Common perception is that energy intake should be limited before sleep as previous research has shown that satiety in response to meals( Reference de Castro 1 , Reference de Castro 2 ), metabolic rate and glucose tolerance( Reference Katayose, Tasaki and Ogata 3 – Reference Van Cauter, Desir and Decoster 5 ) decrease with the time of day, which may lead to overeating and negatively affect cardiometabolic health and body composition. Indeed, data from studies on shift workers( Reference Macagnan, Pattussi and Canuto 6 , Reference Lennernas, Akerstedt and Hambraeus 7 ) and those with night eating syndrome( Reference Gluck, Venti and Salbe 8 – Reference Marshall, Allison and O'Reardon 10 ) provide evidence for an increased cardiometabolic risk with night-time eating. In the absence of data from studies on shift workers and night eating syndrome, much of the data regarding night-time eating stem from animal models( Reference Arble, Bass and Laposky 11 ) and epidemiological studies( Reference de Castro 1 , Reference Andersen, Stunkard and Sorensen 12 – Reference Tholin, Lindroos and Tynelius 14 ). There are even fewer intervention studies demonstrating a link between night-time food intake, cardiometabolic risk factors and weight regulation. Furthermore, the adverse findings from epidemiological studies have been observed in response to large mixed meals. Thus, it remains unclear whether altering food choice (i.e. consuming small nutrient-dense, low-energy, foods or single macronutrients) would alter the trend for increased cardiometabolic risk and unfavourable body composition.
To our knowledge, only one recent intervention study( Reference Hibi, Masumoto and Naito 15 ) has reported significant, albeit small, decreases in 24 h fat oxidation and increases in total cholesterol and LDL-cholesterol levels in women consuming a night-time v. daytime snack (837 kJ (200 kcal), approximately 20 g carbohydrate, approximately 3 g protein and approximately 11 g fat) for 13 d. Although the blood lipid values were within normal ranges, this study indicated that late-night eating might increase cardiometabolic risk( Reference Hibi, Masumoto and Naito 15 ). In contrast, Waller et al. ( Reference Waller, Vander Wal and Klurfeld 16 ) reported that consuming small snacks (approximately 502 kJ (120 kcal); cereal: 23–32 g carbohydrate, 2–6 g protein and < 0·5 g fat, with two-third cup of fat-free milk) in the evening after dinner reduced total daily energy intake and facilitated weight loss in overweight and obese adults. This study( Reference Waller, Vander Wal and Klurfeld 16 ) highlights the potential health benefits of night-time eating if the composition of the food is lower in energy and higher in protein. Notably, both these studies opted for a higher-carbohydrate snack as opposed to a higher-protein meal, which has been shown to reduce cardiometabolic risk( Reference Aude, Agatston and Lopez-Jimenez 17 – Reference Arciero, Ormsbee and Gentile 19 ).
Interestingly, two acute overnight studies carried out by the same laboratory( Reference Groen, Res and Pennings 20 , Reference Res, Groen and Pennings 21 ) have demonstrated positive physiological responses to night-time protein intake. These studies have shown that casein (CAS) protein (40 g) consumed 30 min before sleep( Reference Res, Groen and Pennings 21 ) or administered during sleep via nasogastric tubing( Reference Groen, Res and Pennings 20 ) increases overnight muscle protein synthesis in recreationally active young and elderly men, respectively. Appetite the following morning was also found to be reduced in the elderly men( Reference Groen, Res and Pennings 20 ). Although CAS protein is often recommended to be consumed just before bedtime due to its slow release( Reference Boirie, Dangin and Gachon 22 ), published data from studies comparing the effects of protein type (whey (WH) v. CAS) and those of different macronutrients (carbohydrate v. protein) consumed at night before sleep on physiological outcomes in various populations are scarce. Our laboratory has recently shown that consuming a single 30 g serving of WH, CAS or carbohydrates (586–628 kJ or 140–150 kcal) 30 min before sleep exerts more favourable effects on resting metabolism the following morning (8–10 h later) compared with consuming a non-energy placebo in physically active men( Reference Madzima, Panton and Fretti 23 ). However, the impact of night-time feeding in overweight or obese people has not been explored, despite concern regarding night-time eating in these individuals( Reference Ceru-Bjork, Andersson and Rossner 24 ). Furthermore, no study has examined cardiometabolic (insulin, leptin and adiponectin), anabolic (insulin-like growth factor-1, IGF-1), catabolic (cortisol) or inflammatory (high-sensitivity C-reactive protein, hs-CRP) markers in response to night-time macronutrient intake. Therefore, the purpose of the present study was to determine whether night-time ingestion of a low-energy (586–628 kJ or 140–150 kcal) protein beverage before sleep could acutely alter appetite or cardiometabolic risk the following morning (8–10 h later) compared with night-time ingestion of a carbohydrate beverage. In addition, the effect of protein type (WH v. CAS) was also investigated. We hypothesised that the ingestion of a low-energy protein drink would elicit favourable appetite and cardiometabolic changes compared with the ingestion of a carbohydrate placebo (PLA) in overweight and obese women.
Methods
Participants
A total of forty-nine sedentary ( < 2 × per week within the past 6 months), overweight or obese (BMI: 25·7–54·6 kg/m2), but otherwise healthy women aged 18–45 years were recruited for the present study. Participants were excluded if they had uncontrolled hypertension (blood pressure (BP) >160/100 mmHg), were taking BP or cholesterol medications, had been diagnosed with CVD, stroke, diabetes, or thyroid or kidney dysfunction, were heavy smokers (>10 cigarettes/d), took nutritional supplements (except for a multivitamin), or had any allergies to milk products. All the participants were pre-screened via the telephone to ensure their eligibility and were asked to refrain from smoking, consuming caffeine and performing physical activity 24 h before each visit. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Florida State University Human Subjects Institutional Review Board. Written informed consent and medical history forms were completed by all the participants and obtained from them before participation.
Study design
This was a stratified, randomised, double-blind acute study. The participants reported to the laboratory fasted between 06.00 and 09.00 hours for measurements and assessments (discussed below) on two different occasions (visit 1 and visit 2). Anthropometric parameters, heart rate and BP were only measured during visit 1, while appetite and RMR were measured and blood samples collected during both visits.
Visit 1 (baseline)
Measurement of anthropometric parameters
Height and body weight were measured using a wall-mounted stadiometer and a digital scale (SECA), respectively. Body composition was determined by dual-energy X-ray absorptiometry (model DPX-IQ, GE Medical Systems) with the participants in the supine position as described previously( Reference Arciero, Gentile and Martin-Pressman 25 ).
Measurement of heart rate and blood pressure
After 5 m of seated rest, resting heart rate and BP were measured twice by the same technician, and the average of the two measurements was recorded to verify whether the participants met the inclusion criteria. Heart rate was measured manually at the radial artery for 60 s. BP was measured using a manual sphygmomanometer (American Diagnostic Corporation) and a stethoscope.
Assessment of appetite
Appetite was assessed using the visual analogue scale( Reference Flint, Raben and Blundell 26 ). The visual analogue scale is a 100 mm horizontal scale with opposing extremes of each appetite sensation (hunger, satiety and desire to eat) anchored at each end of the 100 mm line (‘not at all’ to ‘extremely’). The participants rated their subjective feelings, at that moment, by placing a vertical line along the 100 mm scale, and these ratings were converted to a score in millimetres using a standard millimetre ruler. Higher scores indicated greater feelings of each sensation( Reference Flint, Raben and Blundell 26 ). All the measurements were taken in the morning.
Metabolic testing
RMR and respiratory quotient were measured via indirect calorimetry using a mouthpiece and a nose clip (ParvoMedics TrueOne 2400 metabolic cart)( Reference Roffey, Byrne and Hills 27 ) with the participants resting supine on a bed in a dark, quiet and climate-controlled isolated room (20–23°C). Before testing, duplicate calibrations were performed on a flow meter using a 3-litre syringe and on gas analysers using verified gases of known concentrations. Thereafter, gas exchange was measured continuously for 30 min and measurements recorded in the last 20 min were used for data analysis.
Blood sampling and analysis
In the morning during visits 1 and 2, two fasting venous blood samples (20 ml total) were collected 10 min apart from the antecubital vein into vacutainer tubes (Becton, Dickinson & Company). Whole blood was subsequently analysed for TAG, total cholesterol, HDL-cholesterol, LDL-cholesterol and glucose (Cholestech LDX)( Reference Dale, Jensen and Krantz 28 ). The remaining samples were centrifuged (IEC CL3R Multispeed Centrifuge, Thermo Electron Corporation) for 15 min at 3500 rpm at 4°C. Serum aliquots of 300 μl were transferred into microtubes and stored at − 80°C for later batch analysis of insulin, leptin, adiponectin, hs-CRP, IGF-1 and cortisol. All assays were performed in duplicate in a single assay using commercially available ELISA kits according to the manufacturers' instructions (insulin and hs-CRP: IBL International, Inc.; all other hormones: R&D Systems, Inc.). The average of duplicate samples was used for statistical analyses. The intra-assay CV for insulin, leptin, adiponectin, hs-CRP, IGF-1 and cortisol were 3·7, 4·8, 7·7, 3·1, 6·8 and 9·7 %, respectively. The inter-assay CV for insulin, leptin, adiponectin, hs-CRP, IGF-1 and cortisol were 18·5, 6·8, 11·3, 4·1, 10·9 and 21·6 %, respectively. The homeostatic model assessment of insulin resistance (HOMA-IR)( Reference Matthews, Hosker and Rudenski 29 ) value was determined using the following equation:
Visit 2 (post testing)
During visit 2, appetite and RMR were measured and blood samples collected as described above. Visit 2 was conducted at least 48 h but no more than 1 week after visit 1 at the same time of day (06.00–09.00 hours) following night-time intake of the assigned supplement.
Nutritional supplementation
After being stratified by body fat percentage during visit 1, the participants were randomly assigned to one of the three groups: WH (n 19; 628 kJ (150 kcal); 30 g WH protein (50 % blend of WH protein isolate and concentrate), 4 g carbohydrate and 1·5 g fat); CAS (n 16; 586 kJ (140 kcal); 30 g micellar CAS protein, 3 g carbohydrate and 0·5 g fat); PLA (n 14; 628 kJ (150 kcal); 0 g protein, 34 g maltodextrin and 2 g fat). The supplements were provided in powdered form and contained small amounts of Na, K and Ca for consistency and flavouring. To maintain blindness between the research personnel and participants, all supplement powders were identical in appearance, flavour (vanilla chai) and texture and were pre-labelled and packaged as A, B and C by an external investigator. The serving size of each powdered supplement was 38 g, and each powdered supplement was measured using a digital gram scale (Model 7224DA, Allied Fisher Scientific) and distributed in single-serving plastic quart-sized bag. The participants were asked to return their empty supplement bag or verbally comply that they consumed their supplement at the appropriate time. A non-energy placebo was not included in the present study to ensure that the participants were unaware of the supplement they were receiving to maintain compliance. The participants were provided with shaker bottles and instructed to mix their powdered supplement with 12 oz of water and consume this mixture as the last food or energy beverage of the day at least 2 h after dinner but no more than 30 min before sleep the night before visit 2. The participants were asked to maintain their habitual food intake for both laboratory visits.
Statistical analyses
A one-way ANOVA was used to determine whether there were differences among the groups at baseline. A 3 × 2 (group × time) repeated-measures ANOVA was used to examine differences in the dependent variables. Tukey's post hoc analysis was carried out to detect differences when significant main effects were observed. JMP Pro10 statistical software (SAS Institute, Inc.) was used for all the analyses. Values reported are means with their standard errors, unless otherwise specified.
Results
Participants
In the present study, 238 individuals were pre-screened via telephone, eighty-two met the eligibility criteria, and forty-nine completed baseline testing. Of these forty-nine women, forty-four completed the two visits (WH: n 16; CAS: n 15; PLA: n 13) and five women did not show up for visit 2 due to scheduling conflicts. The starting date of the last menstruation was recorded for each of the participants to determine the phase of the menstrual cycle that they were in during laboratory testing using a 28 d cycle with ovulation at day 14. All the participants were tested during the follicular phase (WH: n 9; CAS: n 4; PLA: n 5) or the luteal phase (WH: n 6; CAS: n 7; PLA: n 7) of their menstrual cycle, and some reported not having menstrual cycle due to Depo-Provera® use (WH: n 1; CAS: n 3; PLA: n 1) and hysterectomy (CAS: n 1). There were no differences in any of the anthropometric parameters, body composition, BMI, or resting heart rate and BP among the groups at baseline (Table 1). Among the participants, two, one in each protein group, were smokers (0·5 cigar and 5–7 cigarettes/d, respectively). Separate data analyses indicated no significant differences when the data of these participants were included or excluded from the dataset and, therefore, their data were included while reporting the final results.
Table 1 Characteristics of the study participants (Mean values and standard deviations; n 44)
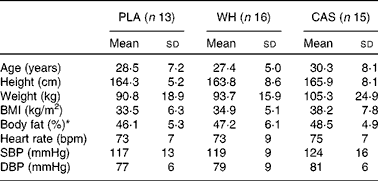
PLA, carbohydrate placebo; WH, whey; CAS, casein; SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Body fat was assessed by dual-energy X-ray absorptiometry.
Appetite
No group × time interactions were observed for any of the appetite sensations (hunger, satiety and desire to eat; P>0·05). However, there was a main effect of time, indicating that night-time macronutrient intake increases satiety (P= 0·03) and reduces desire to eat (P= 0·006) the following morning (Fig. 1).
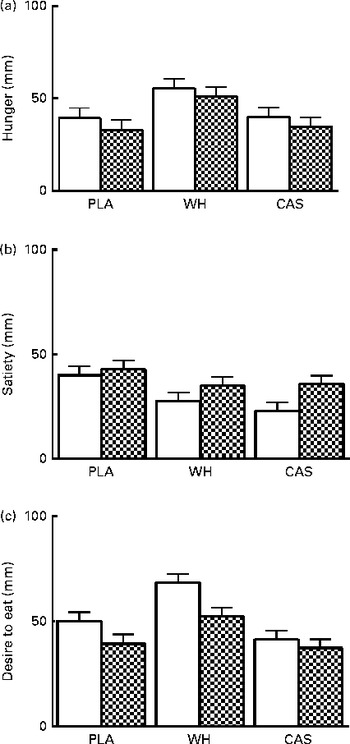
Fig. 1 Subjective appetite ratings for (a) hunger, (b) satiety and (c) desire to eat. Values are means, with their standard errors represented by vertical bars. There was a main effect of time for satiety and desire to eat (P< 0·05). Visit 1 (baseline, □), morning before night-time nutrient intake; visit 2 (post testing, ![]() ), morning after night-time nutrient intake; PLA, carbohydrate placebo; WH, whey; CAS, casein.
), morning after night-time nutrient intake; PLA, carbohydrate placebo; WH, whey; CAS, casein.
Metabolic testing
No time or group × time interactions were observed for resting metabolism (WH – baseline: 7729 (se 314) kJ/d v. post: 7813 (se 314) kJ/d; CAS – baseline: 8709 (se 314) kJ/d v. post: 9190 (se 314) kJ/d; PLA – baseline: 8654 (se 331) kJ/d v. post: 8127 (se 314) kJ/d; P>0·05) or respiratory quotient (WH – baseline: 0·82 (se 0·01) v. post: 0·85 (se 0·01); CAS – baseline: 0·84 (se 0·01) v. post: 0·86 (se 0·01); PLA – baseline: 0·87 (se 0·01) v. post: 0·86 (se 0·01); P>0·05) (Fig. 2).

Fig. 2 Changes in the percentage of (a) respiratory quotient and (b) RMR. Values are means, with their standard errors represented by vertical bars. PLA, carbohydrate placebo; WH, whey; CAS, casein.
Blood variables
Fasting blood lipid and hormone values following night-time macronutrient intake are given in Tables 2 and 3, respectively. The sample sizes differed slightly among the biomarker assays because some participants' samples were not useable or drawing of blood was not possible. Blood lipid and glucose values were within normal ranges and no time or group × time interactions were observed (P>0·05). A main effect of time, suggesting an increase in insulin levels (P= 0·004) and HOMA-IR (P= 0·01) was observed but there were no differences among the groups. No significant differences were observed in serum adiponectin, cortisol, leptin, total IGF-1 and hs-CRP levels. Notably, hs-CRP levels were elevated above the clinical range (>1 mg/l)( Reference Pearson, Mensah and Alexander 30 ) in all the participants at baseline and after the intervention.
Table 2 Blood lipid and glucose levels (Mean values with their standard errors)
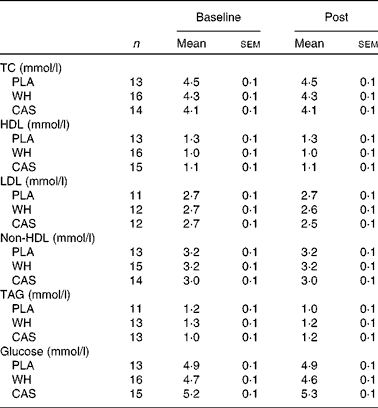
TC, total cholesterol; PLA, carbohydrate placebo, WH, whey; CAS, casein.
Table 3 Hormonal responses to night-time macronutrient intake (Mean values with their standard errors)
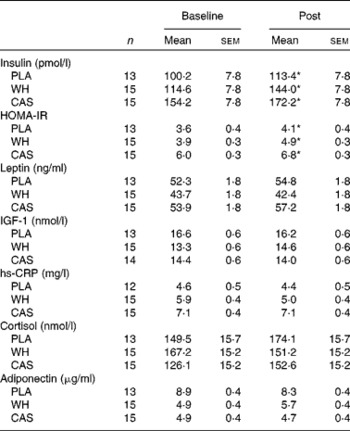
PLA, carbohydrate placebo; WH, whey; CAS, casein; HOMA-IR, homeostatic model assessment of insulin resistance; IGF-1, insulin-like growth factor-1; hs-CRP, high-sensitivity C-reactive protein.
* There was a main effect of time (P< 0·05).
Discussion
Despite the common perception that eating in the late evening is detrimental to cardiometabolic health, limited empirical evidence supports this contention( Reference de Castro 1 , Reference Arble, Bass and Laposky 11 – Reference Tholin, Lindroos and Tynelius 14 ) in populations independent of those whose daily energy intake is highest at night (i.e. night shift workers and those with night eating syndrome). Moreover, these preliminary studies have typically used large mixed meals rather than smaller, nutrient-dense macronutrients. The present study investigated whether protein or carbohydrate consumed as a single macronutrient at night before sleep could acutely alter appetite and cardiometabolic risk the following morning in overweight and obese women. We hypothesised that the ingestion of a protein drink would elicit favourable appetite and cardiometabolic changes compared with the ingestion of a PLA. The primary findings of the present study suggest that when a small serving of carbohydrate or protein is consumed at night before going to bed, insulin resistance is increased along with subjective feelings of satiety and reduced desire to eat the following morning. Therefore, we reject our hypothesis.
Traditional practice has been to limit energy intake in the late evening as metabolism has been reported to slow during sleep( Reference Katayose, Tasaki and Ogata 3 , Reference Sato, Nakamura and Ogata 4 ) and may therefore promote energy storage. Interestingly, higher daily protein intake has been shown to attenuate the typical drop in sleep-time metabolism compared with the intake of diets higher in carbohydrate and fat( Reference Whitehead, McNeill and Smith 31 ). Moreover, dietary proteins have a greater thermic effect compared with other macronutrients( Reference Arciero, Ormsbee and Gentile 19 , Reference Whitehead, McNeill and Smith 31 – Reference Westerterp, Wilson and Rolland 34 ), and higher-protein diets have been shown to result in a significantly higher sleeping metabolic rate compared with lower-protein diets( Reference Lejeune, Westerterp and Adam 35 ). Of interest is whether these alterations in sleeping metabolic rate in response to proteins could potentially translate into greater increases in morning metabolism compared with sleeping metabolic rate in response to carbohydrates. Interestingly, our laboratory has recently shown that, regardless of the macronutrient type, consuming 586–628 kJ (140–150 kcal) of protein or carbohydrate 30 min before sleep increases morning metabolism in healthy physically active men compared with consuming a non-energy placebo, while no statistically significant differences have been observed between the carbohydrate and protein groups( Reference Madzima, Panton and Fretti 23 ). As has been mentioned in the Methods section, we decided not to include a non-energy placebo in the present study as all the supplements used were identical in appearance and flavour to ensure that the participants were unaware of the supplement they were receiving and to maintain compliance. Regardless, these findings suggest that acute night-time protein or carbohydrate intake does not negatively affect morning metabolism. Interestingly, although not statistically significant (P= 0·30), the direction of change for morning metabolism was positive for the protein groups and negative for the placebo group (Fig. 2(b)). Subsequent studies that investigate night-time eating over a longer duration may be able to tease out differences and promote suggestions for specific foods to be eaten at night before going to bed. Using a mixed-meal feeding regimen at night, it has been suggested that this may not hold true with a slightly longer-duration intervention. After a 13 d intervention, Hibi et al. ( Reference Hibi, Masumoto and Naito 15 ) reported lower 24 h fat oxidation (measured in a metabolic chamber) in normal-weight women who consumed an approximately 837 kJ (200 kcal) snack (approximately 20 g carbohydrate, approximately 3 g protein and approximately 11 g fat) at night (23.00 hours) compared with that in women who consumed their snack during the day (10.00 hours). Of note, it is difficult to compare the night-time feeding studies that do exist because of the different macronutrient profiles that are provided to participants in each study. It is also clear that extension of night-time eating durations in future research is warranted and may yield different findings. Nevertheless, our data indicate that while there is no statistically significant change in RMR, there are magnitude inferences that exist in the study participants in response to acute night-time protein or carbohydrate intake.
Night-time intake of carbohydrate or protein did not alter fasting blood glucose or lipid values the following morning, which were all within normal ranges with the exception of LDL-cholesterol values. These levels were at the upper end or slightly above the optimal values in the study participants. The study carried out by Hibi et al. ( Reference Hibi, Masumoto and Naito 15 ) reported higher total cholesterol and LDL-cholesterol values with night-time snacking compared with those observed with daytime snacking, but all these values were within normal ranges. Sato et al. ( Reference Sato, Nakamura and Ogata 4 ) compared the effect of a standardised meal (3021 kJ (722 kcal); 63·3 % carbohydrate, 19·9 % fat and 16·8 % protein) consumed at 19.00 or 22.30 hours on blood glucose levels and reported that fasting blood glucose levels the following morning were similar between meal times; however, postprandial glucose levels after breakfast were higher as a result of the late-evening meal. This finding is supported by other studies that utilised a high-carbohydrate food option( Reference Tsuchida, Hata and Sone 36 ). Interestingly, despite the lack of change in fasting glucose levels, an effect of time suggesting an increase in fasting insulin levels and HOMA-IR was observed. This suggests that night-time ingestion of protein or carbohydrate before sleep may elicit unfavourable metabolic effects the following morning in sedentary overweight and obese women. In fact, compared with their lean counterparts, obese individuals tend to have higher fasting insulin levels( Reference Daghestani, Ozand and Al-Himadi 37 ). In obese adults, fasting insulin levels appear to have the greatest effect on HOMA-IR, which is the product of glucose and insulin divided by a normalised factor( Reference Matthews, Hosker and Rudenski 29 ), as demonstrated by the lack of changes in glucose levels reported herein and elsewhere( Reference Vogeser, Konig and Frey 38 ). It has been suggested that a HOMA-IR value >2·5 is indicative of insulin resistance( Reference Matthews, Hosker and Rudenski 29 ), and others( Reference Vogeser, Konig and Frey 38 ) and we have demonstrated that obese individuals are insulin resistant based on this model. Fortunately, exercise training has been shown to reduce fasting insulin levels( Reference Martins, Kulseng and King 39 ), which may also reduce HOMA-IR. Therefore, although we observed an increase in insulin levels and HOMA-IR acutely, longer-duration interventions that combine night-time eating with perhaps daily exercise may alter this effect.
With the exception of insulin, night-time macronutrient intake did not influence morning hormonal responses. Martens et al. ( Reference Martens, Rutters and Lemmens 40 ) examined the role of single macronutrients consumed as beverages in the afternoon on cortisol secretion and reported higher postprandial cortisol levels in the carbohydrate beverage group compared with the water control group, while no differences existed among the protein, fat and control groups. To our knowledge, no study has examined hormonal responses the following morning after night-time macronutrient intake, and the paucity of available data on this practice warrants further investigation with longer-duration intervention studies. Although we did not find differences in hormonal responses among the groups, our data do add to current research demonstrating that overweight and obesity are associated with hyperleptinaemia and inflammation (as measured by hs-CRP levels), as the values recorded in the present study were similar to those reported by other studies carried out in obese adults( Reference Visser, Bouter and McQuillan 41 – Reference Sinha, Opentanova and Ohannesian 43 ). Nevertheless, in the present study, only morning insulin levels and HOMA-IR were affected by night-time eating.
From an acute perspective, the majority of studies comparing the satiating effects of different macronutrients on appetite have reported that proteins are more satiating than carbohydrates when assessed postprandial within 15–270 min (15 min–4·5 h)( Reference Bowen, Noakes and Trenerry 44 – Reference Latner and Schwartz 47 ). However, studies examining the effect of macronutrients consumed at night on appetite the following morning are limited. Moreover, some evidence suggests that the digestion and absorption rates of different proteins can affect their satiating capacity( Reference Hall, Millward and Long 45 , Reference Latner and Schwartz 47 ). An interesting finding of the present study was that consuming a liquid protein or carbohydrate beverage containing 586–628 kJ (140–150 kcal) at night before going to bed reduced subjective appetite, characterised by increased satiety and reduced desire to eat, the following morning (8–10 h later) in sedentary overweight and obese women. In a recent study carried out in our laboratory using a cross-over design( Reference Madzima, Panton and Fretti 23 ), no differences were observed in morning appetite in physically active men consuming WH protein, CAS protein, a carbohydrate (supplements identical to those used in the present study), or a non-energy placebo the previous night. In contrast, Groen et al. ( Reference Groen, Res and Pennings 20 ) demonstrated that administering 40 g of CAS protein (669 kJ (160 kcal)) via nasogastric tubing during sleep (02.00–02.05 hours) lowered subjective hunger ratings the following morning (approximately 5 h later) compared with administering a non-energy (water) placebo in elderly men. Interestingly, despite this difference, there were no objective differences in subsequent food intake at breakfast( Reference Groen, Res and Pennings 20 ). It is likely that increased morning satiety in the sedentary overweight and obese participants in the present study manifested as a compensatory response to night-time intake of energy; however, actual food intake in the morning was not measured. Therefore, the increase in morning satiety is interesting; however, we can only speculate that this response would promote a long-term energy deficit. Morning anorexia is typically reported in patients with night eating syndrome as in these individuals energy intake is highest in the evening( Reference Gluck, Geliebter and Satov 48 ). However, as we asked the study participants to consume their respective supplements at least 2 h after dinner but no more than 30 min before going to bed and only provided 586–628 kJ (140–150 kcal), the increase in morning satiety is probably not a result of overeating the previous night and may potentially help to prevent overeating in these individuals. In a 4-week study carried out by Waller et al. ( Reference Waller, Vander Wal and Klurfeld 16 ), a reduction in total daily energy intake and weight ( − 0·84 (sd1·62) kg) was observed in overweight and obese adults who consumed ready-to-eat cereals (502 kJ (120 kcal)) with skimmed milk at least 90 min after dinner. Interestingly, recent data have indicated that when consuming a small (837 kJ (200 kcal)) v. large (2929 kJ (700 kcal)) evening meal when on a 12-week isoenergetic weight-loss diet (5858 kJ (1400 kcal)), overweight and obese women with the metabolic syndrome lost more body weight and body fat and had a better cardiometabolic profile( Reference Jakubowicz, Barnea and Wainstein 49 ). Thus, it is possible that when the food size (total energy) and choice are appropriate, night-time snack intake may have positive health outcomes in the long term (as opposed to acute), hence contradicting popular belief.
The present study has a few limitations that need be addressed. As a result of manufacturing to ensure equal appearance, flavour and texture, the protein supplements used in the present study differed slightly in their energy content (CAS: 586 kJ (140 kcal); WH: 628 kJ (150 kcal)) and subtle differences in sensory characteristics of the supplements might have existed. In addition, it is possible that the final mixed-macronutrient meal of the day might have affected the night-time supplement. However, as there were no differences among the groups with regard to the measured variables, specifically satiety and resting metabolism (only an effect of time was observed), the small difference in energy content among the supplements did not affect the results of the present study. In addition, we did not measure caffeine intake in the present study and it is possible that the abstinence period before metabolic testing might have influenced our findings. The results of the present study should be interpreted with caution, as we did not include a non-energy placebo in order to maintain the double-blind design of the study.
Conclusion
The present study is the first to investigate whether protein or carbohydrate consumed as a single macronutrient at night before sleep can acutely alter appetite and cardiometabolic risk the following morning in sedentary overweight and obese women. Our data indicate that consuming 586–628 kJ (140–150 kcal) of protein or carbohydrate before sleep increases satiety and reduces the desire to eat the following morning. We also found, albeit non-significant, improvements in RMR with protein consumption but not with PLA consumption. However, increases in insulin levels and insulin resistance were also observed with this acute night-time eating protocol. Therefore, protein or carbohydrate consumption before sleep may increase cardiometabolic risk but may favourably affect appetite in sedentary obese women. Of note is that data published previously by our laboratory( Reference Madzima, Panton and Fretti 23 ) and others( Reference Res, Groen and Pennings 21 ) indicate that in non-sedentary lean populations, night-time eating appears to be helpful or inconsequential for health and metabolism. Although some recent data exist( Reference Figueroa, Wong and Kinsey 50 ), more research addressing the combination of night-time eating and exercise training and of longer durations is needed.
Acknowledgements
The authors thank Charles Blay, Yasmine Kahok, Celeste Heilman and Emily Mattei for their help with participant recruitment and data collection. They also thank the volunteers for their dedication and participation in the present study.
The present study was supported by funding from the Florida State University.
The authors' contributions are as follows: M. J. O. conceived and designed the study, secured funding for the project, oversaw data collection and analysis and manuscript preparation; A. W. K. and W. R. E. carried out participant recruitment and data collection and analysis and assisted with manuscript preparation; T. A. M. assisted with data collection and manuscript preparation; P. J. A. helped with manuscript preparation; L. B. P. and J.-S. K. helped with the study design and manuscript preparation. All authors read and approved the final manuscript.
The authors have no financial or other interests concerning the outcomes of the investigation.







