Depression is a common mental disorder and is very common in older persons, especially those affected by medical illness( Reference Beekman, Geerlings and Deeg 1 , Reference Alexopoulos 2 ). Its prevalence and highly recurrent nature make it a major contributor to the global burden of disease. It is estimated to affect more than 350 million people worldwide, and this prevalence is expected to increase in the future( 3 ). Therefore, the prevention of depression is a high priority for public health.
There are indications that diet may play a protective role in the development, progression and treatment of depression and depressive symptoms( Reference Lopresti, Hood and Drummond 4 – Reference Opie, O’Neil and Itsiopoulos 6 ). Previous studies have suggested that people suffering from depressive symptoms have higher levels of inflammation and oxidative stress than those without depressive symptoms( Reference Howren, Lamkin and Suls 7 – Reference Vavakova, Durackova and Trebaticka 9 ). The nutrients EPA and DHA( Reference Grosso, Pajak and Marventano 10 – Reference Bloch and Hannestad 12 ), and folate( Reference Sanchez-Villegas, Doreste and Schlatter 13 – Reference Murakami, Mizoue and Sasaki 15 ), Mg( Reference Derom, Sayon-Orea and Martinez-Ortega 16 – Reference Tarleton and Littenberg 18 ) and Zn( Reference Vashum, McEvoy and Milton 19 – Reference Miki, Kochi and Eguchi 21 ) might have anti-inflammatory properties and appear to be associated with lower depressive symptoms. However, it is difficult to draw conclusions from associations between individual nutrients or foods because people eat meals consisting of several foods with complex combinations of nutrients that are likely to be synergistic or interactive( Reference Akbaraly, Brunner and Ferrie 22 ).
Owing to this fact, studies within several cohorts have examined overall dietary patterns in relation to depressive symptoms with the aim to assess habitual diet as one overall exposure( Reference Akbaraly, Brunner and Ferrie 22 – Reference Skarupski, Tangney and Li 32 ). The Mediterranean dietary pattern (MDP) is the most commonly investigated dietary pattern and consists of a high ratio of MUFA:SFA, high intakes of fruits and nuts, vegetables, fish, legumes and cereals, moderate intakes of alcohol, milk and dairy products and low intakes of meat and meat products( Reference Trichopoulou, Costacou and Bamia 33 ). This pattern has been inversely associated with depression in several cohort studies( Reference Sanchez-Villegas, Delgado-Rodriguez and Alonso 26 , Reference Hodge, Almeida and English 28 , Reference Skarupski, Tangney and Li 32 ).
To date, most studies have used hypothesis-driven quality scores (a priori) or approaches that are driven by the underlying dietary data (a posteriori) to derive dietary patterns in relation to depression. The a priori approach such as the MDP has the disadvantage that it only includes foods that are hypothesised to have an effect on health and may miss other relevant foods. A posteriori methods such as cluster analysis and principal component analysis have the limitation that they describe the prevailing dietary patterns in a population, and this may not be associated with the disease outcome under study( 34 ).
A hybrid approach, reduced rank regression (RRR), shows complementary value in identifying dietary patterns( Reference Ocke 35 , Reference Hoffmann, Schulze and Schienkiewitz 36 ). The great advantage of RRR is that it creates a dietary pattern that is associated with the intake of nutrients known to be related to the outcome measure. In this way, the dietary pattern produced is more specific to the outcome of interest( Reference Schulze and Hoffmann 37 ) in that it identifies the combinations of food intake that explain the most variation in risk for a specific disease( 34 ). Investigating dietary patterns, obtained by RRR, in relation to depressive symptoms using repeated measurements and continuous scores may provide new insights into the relationship between diet and depression.
Therefore, the aim of the present study was to identify dietary patterns using RRR and to explore their associations with depressive symptoms over 9 years in the Invecchiare in Chianti (InCHIANTI) study. Our hypothesis is that participants who adhere to the dietary pattern derived by RRR report fewer depressive symptoms.
Methods
Participants and study design
The InCHIANTI study is an ongoing Italian population-based cohort study, which is performed in two sites in Tuscany, Italy (Greve in Chianti and Bagno a Ripoli). Baseline data were collected from 1998 until 2000. At baseline, 1453 people were randomly recruited for the study using a multistage sampling method, and included participants were aged between 20 and 102 years. Participants were excluded from the analysis when baseline data for diet or depressive symptoms were missing (n 91). In total, 1362 participants were included for the analyses. A home interview was conducted, where participants were asked to answer questionnaires concerning lifestyle, diet and depression. Next, they were invited to the study location for a clinical examination within 21 d after the home interview. Follow-up data were collected after 3, 6 and 9 years (from 2001–2003, 2004–2006 and 2007–2009, respectively). The study protocol has been described in more detail previously( Reference Ferrucci, Bandinelli and Benvenuti 38 ). The ethics committee of the Italian National Institute of Research and Care on Aging approved the study protocol, and informed consent was obtained from all individual participants included.
Depressive symptoms assessment
The Centre for Epidemiologic Studies Depression (CES-D) scale was used to detect depressive symptoms at baseline and at all follow-ups (after 3, 6 and 9 years)( Reference Radloff 39 ). The questionnaire was translated into Italian and was completed by the participants during the home interview( Reference Ferrucci, Bandinelli and Benvenuti 38 ). The CES-D is a widely used self-report scale that consists of twenty items (scores range from 0 to 60 points) and has been illustrated to be a valid instrument for identifying depressive symptoms in older adults( Reference Beekman, Deeg and Van Limbeek 40 ) and in Italians( Reference Fava 41 ). In this study, the continuous CES-D scores were used as outcome measures. CES-D scores were log-transformed when not normally distributed. However, for descriptive purposes, CES-D scores ≥20 points were used to define participants with prevalent depressive symptoms at baseline. This cut-off point was used as it has been shown to have high specificity in identifying depression in Italians( Reference Fava 41 ) and older adults( Reference Beekman, Deeg and Van Limbeek 40 ) in previous studies. In addition, in order to gain insight into the impact of participants with major depressive symptoms on the studied association, we also conducted sensitivity analysis using the definition major depressive symptoms (CES-D scores ≥25). This cut-off point has been previously tested in an older population and has been shown to have satisfactory validity (85 %) and specificity (64 %) in identifying major depressive disorders( Reference Haringsma, Engels, Beekman and Spinhoven 42 ).
Dietary pattern assessment
Usual dietary intake information was obtained using a country-specific validated FFQ of North-Central Italy from the European Prospective Investigation into Cancer and Nutrition study containing 248 questions with 188 different food items( Reference Riboli, Hunt and Slimani 43 , Reference Pala, Sieri and Palli 44 ). The validation procedure for the FFQ has been described elsewhere( Reference Pisani, Faggiano and Krogh 45 ). Baseline dietary patterns were identified using RRR, which defines linear combinations of food groups (so called predictors) that maximally explain the variation in a set of response variables. These response variables were considered intermediate markers of depressive symptoms and included intakes of fish fatty acids EPA+DHA (g/d), folate (μg/d), Mg (mg/d) and Zn (mg/d) from the FFQ. EPA+DHA were chosen instead of total n-3 fatty acids because EPA+DHA only contribute a small portion to total n-3 fatty acids intake. A maximum of four factors can be extracted from RRR because we used four response variables, and the successive extracted scores are uncorrelated because the eigenvectors are always orthogonal. The RRR method (including the SAS code) has been described in detail by Hoffman et al.( Reference Hoffmann, Schulze and Schienkiewitz 36 ). As predictors, thirty-one food groups were identified from the FFQ according to their nutritional composition and usual intake based on the Italian National Food Consumption Data in Italian adults (Table 1). Response variables were log-transformed when not normally distributed. Primarily, continuous factor scores were used for all analyses. However, to make factor scores more interpretable, continuous scores were transformed into quartiles, with higher quartiles indicating better adherence to a dietary pattern represented by the nutrients used as response variables.
Table 1 Food groups from the FFQ for identification of dietary patterns according to their nutritional composition and usual intake in the Invecchiare in Chianti study, Italy

Covariates
Models were adjusted for several covariates that were determined to be potential confounding factors: socio-demographic variables included age (in years), sex, education (in years), marital status (never married, married, widowed/divorced) and socio-economic status (SES) (is your current income adequate for your needs: adequate, barely adequate, inadequate). Behavioural variables included the instrumental activities of daily living scale (IADL) (scores: 0–8), the activities of daily living index (ADL) (scores: 0–6), smoking status (never smoker, former smoker, current smoker), alcohol intake (g/d), BMI (kg/m2), waist circumference (cm), physical activity level over the last year (sedentary (hardly any physical activity and mostly sitting/some walking), light (light exercise 2–4 h/week), moderate to intense (light >4 h/week, moderate >3 h/week or intense walks many times per week)) and energy intake (kJ/d). Health status variables included use of antidepressants (yes or no), hypertension (self-reported and via blood pressure tests) (yes or no), diabetes mellitus type 2 (blood glucose >126 mg/dl) (yes or no) and CVD (yes or no).
Statistical analyses
Frequencies and descriptive statistics were used for categorical (%) and continuous (mean values, standard deviations) variables, respectively, to compare subject characteristics with the presence or absence of depressive symptoms (CES-D scores of≥20). Normal distribution was checked with QQ plots and the Shapiro–Wilk test.
Cross-sectional association between diet and depression
In total, 1362 participants were included in the analyses. Univariable linear regression analyses were performed to test all potential confounding factors. BMI, waist circumference, ADL, hypertension, diabetes and CVD were not included as confounding factors for further analyses because they did not significantly change the association under study (β<10 % change). Subsequently, multivariable linear regression models with regression coefficients and 95 % CI were used to identify cross-sectional associations between dietary patterns and depressive symptoms. Crude analysis was performed followed by adjustment for age, sex, education in years, marital status, SES, IADL, smoking status, alcohol intake, physical activity, energy intake and use of antidepressants.
Prospective association between diet at baseline and depressive symptoms over 9 years
For the analysis, we included participants with at least two measurements of CES-D scores, which left us with 1165 participants. Linear mixed models fit by restricted maximum likelihood (REML) were used, with lowest v. highest quartiles of dietary pattern scores at baseline as exposure and repeated measurements of continuous CES-D scores over a period of 9 years as the main outcome. We used linear mixed models, because this method accounts for the dependency of repeated measurements obtained from the same participant over time. In addition, mixed models allow the use of data from participants lost to follow-up. The models initially included only random intercepts; however, random slopes for covariates that may change over time were added to the model and tested for significant improvement in fit (using likelihood ratio tests (P<0·05)). Ultimately, the random intercept model was used because it demonstrated the best fit compared with models with random slopes. The linear mixed models included a fixed factor for time. This time variable combines the CES-D scores of all four time periods (baseline and after 3, 6 and 9 years of follow-up) and represents the CES-D scores over time. Other fixed factors included were age, sex, education in years, marital status, SES, IADL, smoking status, alcohol intake, physical activity, energy intake and use of antidepressants at baseline. We did not adjust for CES-D scores at baseline in order to avoid taking away part of the time effect. To further explore the influence of age on the association with depression, all analyses were repeated and models were stratified by age (<65 years, ≥65 years).
Sensitivity analyses
In order to gain insight into the impact of participants with major depressive symptoms on the studied association, we also conducted sensitivity analysis by repeating mixed models and by solely including participants with major depressive symptoms (CES-D scores≥25) at any time point. Finally, participants who dropped out or who missed one or more measurements during the 9-year follow-up period were excluded from the analyses. Analyses were conducted using SPSS version 21, except for the dietary pattern analyses, which were performed with SAS version 9.3.
Results
Subject characteristics
At baseline, 1362 participants were analysed, of whom 264 participants (75 % women) were characterised with depressive symptoms according to the CES-D questionnaire when a cut-off point of ≥20 was used. Baseline characteristics of the study participants according to depressive symptoms are presented in Table 2. On average, participants who reported depressive symptoms were older, were less educated, were more likely to be widowed, had higher IADL scores, had lower energy intake levels and were physically less active compared with participants without depressive symptoms.
Table 2 Baseline characteristics of study participants according to depressive symptoms in the Invecchiare in Chianti study, ItalyFootnote * (Mean values and standard deviations; percentages)

IADL, instrumental activities of daily living scale; ADL, activities of daily living index; CES-D, Centre for Epidemiologic Studies Depression.
* Depressive symptoms are defined as CES-D scores of ≥20 points.
Dietary patterns
In total, RRR extracted four factors, which in total explained 24 % of the variation in the food groups and 84 % of the variation in the response variables. Subsequent analyses were performed with quartiles of the first factor obtained by RRR, because it explained the highest amount of variation in food groups and response variables and all nutrients were correlated more or less on a similar level to the dietary pattern, whereas the remaining factor scores only represented one particular nutrient (Table 3). The second factor score represented a diet high in EPA+DHA, the third factor score showed foods high in folate and the fourth factor score comprised a diet high in Mg. To identify the more important food groups, a cut-off point of 0·15 was used( Reference Miki, Kochi and Kuwahara 46 ). However, because all food groups are included in the overall score for the dietary pattern per individual, all food groups contribute to some extent to the dietary pattern with RRR. When using this cut-off point, the pattern from the first factor represented a diet characterised by fish, olive oil, several vegetables, fruit, potatoes, cereals, eggs, wine, red and processed meat, and other sauces (particularly tomato sauce) (Table 3). This dietary pattern reflects a typical Tuscan diet and was therefore labelled as ‘typical Tuscan dietary pattern’.
Table 3 Overview of food groups with factor loadings ≥0·15 as the cut-off point derived by reduced rank regression with the explained variation and correlation coefficients in the Invecchiare in Chianti study, Italy (n 1362)

Cross-sectional analyses
In Table 4, the association is shown between the typical Tuscan dietary pattern and depressive symptoms at baseline. Participants with stronger adherence to this dietary pattern reported fewer depressive symptoms (Q1 v. Q4, model 1: B −5·60; 95 % CI −6·84, −4·37). When adjusting for demographic variables, the association was weakened but remained statistically significant (Q1 v. Q4, model 2: B −2·18; 95 % CI −3·49, −0·87). The association became stronger (Q1 v. Q4, model 3: B −2·74; 95 % CI −4·53, −0·95) after additional adjustment for behavioural variables. Additionally adjusting for antidepressant use did not change the association (Q1 v. Q4, model 4: B −2·77; 95 % CI −4·55, −0·98). When repeating the regression analyses with continuous dietary pattern scores as exposure, similar results were found (Table 4).
Table 4 Cross-sectional association between extreme quartiles of the typical Tuscan dietary pattern and continuous Centre for Epidemiologic Studies Depression scores using multivariable linear regression in the Invecchiare in Chianti study, Italy (Regression coefficients and 95 % confidence intervals; n 1362)
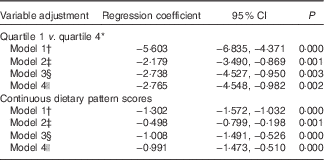
* Quartile 1=−1·84 (sd 0·60), quartile 4=2·25 (sd 1·15).
† Model 1: crude model.
‡ Model 2: adjusted for model 1 and for sex, age, marital status, education in years and socio-economic status.
§ Model 3: adjusted for model 2 and for instrumental activities of daily living scale, smoking status, physical activity, alcohol intake and energy intake.
|| Model 4: adjusted for model 3 and for antidepressant use.
Prospective analyses
Table 5 reveals the association between the typical Tuscan dietary pattern at baseline and depressive symptoms over the 9-year follow-up period. Participants in the highest quartile compared with the lowest quartile of the typical Tuscan dietary pattern at baseline had lower CES-D scores during follow-up (Q1 v. Q4, model 1: B −5·16; 95 % CI −6·22, −4·10). After adjustment for demographic variables, the association became considerably weaker (Q1 v. Q4, model 2: B −0·95; 95 % CI −2·02, 0·12). After additional adjustment for the behavioural variables, the association became stronger again (Q1 v. Q4, model 3: B −1·70; 95 % CI −3·11, −0·29) and did not change with adjustment for antidepressant use (Q1 v. Q4, model 4: B −1·78; 95 % CI −3·17, −0·38). No differences in the association were observed when analysing mixed models with the continuous dietary pattern scores (Table 5).
Table 5 Prospective association between extreme quartiles of the typical Tuscan dietary pattern at baseline and continuous Centre for Epidemiologic Studies Depression scores over time (at baseline and after 3, 6 and 9 years of follow-up) using linear mixed models in the Invecchiare in Chianti study, Italy (Regression coefficients and 95 % confidence intervals; n 1165)
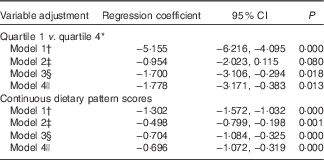
* Quartile 1=−1·74 (sd 0·58), quartile 4=2·35 (sd 1·17).
† Model 1: crude model.
‡ Model 2: adjusted for model 1 and for sex, age, marital status, education in years and socio-economic status.
§ Model 3: adjusted for model 2 and for instrumental activities of daily living scale, smoking status, physical activity, alcohol intake and energy intake.
|| Model 4: adjusted for model 3 and for antidepressant use.
After stratifying for age (<65 years and ≥65 years), participants <65 years revealed stronger associations in the fully adjusted models (Q1 v. Q4, model 4: B −2·96; 95 % CI −5·43, −0·48) compared with the ≥65 years age group (Q1 v. Q4, model 4: B −1·02; 95 % CI −2·64, 0·60). Although stronger associations were found in the younger age group, results were consistent in both groups – that is, higher adherence to the typical Tuscan dietary pattern was associated with lower depressive symptoms.
Sensitivity analyses
For testing the association between diet and major depressive symptoms, the continuous dietary pattern score was used instead of quartiles because of higher power. In participants with major depressive symptoms at any follow-up period (n 323), the association after full adjustment was quite similar as compared with the analysis that included all participants (B −0·71; 95 % CI −1·38, −0·04, P=0·04). Finally, complete data were available for depressive symptoms at all time points for 722 participants. In this sub-group, the observed association was stronger after full adjustment for confounding factors and was statistically significant (Q1 v. Q4: B −1·82; 95 % CI −3·46, −0·17, P=0·03).
Discussion
A diet typically consumed by the Tuscan population – high in vegetables, olive oil, fish, fruits, grains, potatoes, (tomato) sauces, eggs and moderate in wine, red meat and processed meat – was consistently associated with lower depressive symptoms over time in the InCHIANTI cohort. The association between the typical Tuscan dietary pattern and repeated measures of depressive symptoms attenuated after adjustment for confounding factors, but remained statistically significant.
Using RRR to identify dietary patterns and the prospective design of the study with its repeated assessment of depressive symptoms are major strengths of this study. In addition, our study is unique in using continuous scores for depressive symptoms instead of using a cut-off point. This is an advantage because continuous scores are more likely to reflect reality compared with using a cut-off point for depressive symptoms, as the latter could be considered arbitrary. It might be argued that interpreting continuous CES-D scores is difficult; thus, we conducted sensitivity analysis by additionally analysing participants with major depressive symptoms and the results were consistent in this group – a typical Tuscan dietary pattern is associated with lower CES-D scores.
A limitation of the current study is that only baseline dietary pattern was included. This means that participants who developed depressive symptoms during follow-up could have altered their diet and we were not able to capture this. We tested for interaction between baseline diet and time in relation to depressive symptoms, and found it to be not significant, implying that baseline diet remained stable over time. In addition, it might be that baseline diet was influenced by earlier cases of depressive symptoms and that reverse causality is present. However, in the sensitivity analyses, where we stratified on the basis of severity in CES-D scores, the association between the typical Tuscan dietary pattern and depressive symptoms remained stable. Furthermore, using a self-report memory-based method to obtain dietary information is considered as a limitation. The population under study mainly consisted of older individuals and it could be that imprecisions in dietary recall, due to poor cognitive functioning, attenuated the association under study. However, after excluding participants with poor cognitive function (Mini-Mental State Examination (MMSE) scores<24), the association remained unchanged (data not shown). The self-reported subjective CES-D questionnaire that was used for defining depressive symptoms may also be a limitation because it relies on self-reported answers without a clinical diagnosis of depression. However, the questionnaire has been validated extensively and shows good validity( Reference Beekman, Deeg and Van Limbeek 40 , Reference Fava 41 ). Finally, residual confounding cannot be ruled out despite adjustment. Although we adjusted for a number of chronic diseases (hypertension, diabetes, CVD and IADL) in our analyses, this may not cover all conditions that could impact depressive symptoms.
Several cohort studies have investigated the association between dietary patterns in adults and depressive symptoms, using a cut-off point to define depressive symptoms( Reference Akbaraly, Brunner and Ferrie 22 , Reference Chocano-Bedoya, O’Reilly and Lucas 23 , Reference Akbaraly, Sabia and Shipley 25 – Reference Le Port, Gueguen and Kesse-Guyot 30 , Reference Skarupski, Tangney and Li 32 ). Four prospective cohort studies analysed a Mediterranean (like) diet( Reference Akbaraly, Sabia and Shipley 25 , Reference Sanchez-Villegas, Delgado-Rodriguez and Alonso 26 , Reference Hodge, Almeida and English 28 , Reference Skarupski, Tangney and Li 32 ), using a priori methods, and found similar results to the prospective results of the present study. A diet high in vegetables, fruits, cereal grains and fish reduced the risk of having depressive symptoms( Reference Sanchez-Villegas, Delgado-Rodriguez and Alonso 26 , Reference Skarupski, Tangney and Li 32 ) or psychological distress( Reference Hodge, Almeida and English 28 ). Akbaraly et al.( Reference Akbaraly, Sabia and Shipley 25 ) observed that women who improved or adhered to a healthy diet had a lower risk of developing recurrent depressive symptoms. Five observational studies derived dietary patterns using a posteriori methods (principal component analysis or factor analysis). Results from these studies were inconsistent. Two of these studies found that higher intakes of fruit, vegetables, fish and poultry lowered the risk of developing depressive symptoms( Reference Akbaraly, Brunner and Ferrie 22 , Reference Rienks, Dobson and Mishra 27 ). In contrast, three other cohort studies did not find a prospective association between a healthy dietary pattern and depressive symptoms( Reference Chocano-Bedoya, O’Reilly and Lucas 23 , Reference Chan, Chan and Woo 29 , Reference Le Port, Gueguen and Kesse-Guyot 30 ). Two studies found a cross-sectional, inverse association between a diet high in vegetables, fruits and olive oil and depressive symptoms; however, when analysed prospectively, the relationship reduced towards the null( Reference Chan, Chan and Woo 29 , Reference Le Port, Gueguen and Kesse-Guyot 30 ). Finally, a cohort study performed in women found no clear association between a healthy dietary pattern (high in fruits, vegetables, fish, poultry and whole grains) and depressive symptoms( Reference Chocano-Bedoya, O’Reilly and Lucas 23 ).
Unlike the studies mentioned above that used a priori or a posteriori methods to derive dietary patterns, we created a dietary pattern with food groups high in EPA+DHA, folate, Mg and Zn, which have been previously inversely associated with depressive symptoms( Reference Grosso, Pajak and Marventano 10 – Reference Miki, Kochi and Eguchi 21 ). Whereas the dietary patterns described in previous studies consisted mainly of vegetables, fish, olive oil, poultry and whole grains, the typical Tuscan dietary pattern also comprised these elements but additionally included red and processed meat, pasta and bread. Red and processed meat (mainly represented by antipasto misto dishes), pasta and bread are typically eaten foods in Tuscany, Italy, because these products are easily accessible and favoured by the population in the Tuscan area, which may explain their important contribution to the dietary pattern derived. However, despite the high factor loadings for red and processed meat, the actual daily intake was quite low (50 and 27 g/d, respectively), whereas intakes of bread (109 g/d) and pasta (114 g/d) were higher. Thus, on the basis of our results, it appears that moderate intakes of red and processed meat and higher intakes of pasta and bread fit into a dietary pattern that is consistently associated with lower depressive symptoms. Similar results have been observed by Jacka et al.( Reference Jacka FN, Williams and Mann 47 ) who found that moderate intakes of red meat (28–57 g/d) does not increase depressive symptoms.
In the sensitivity analysis, we tested whether our findings might be influenced by depression severity. We observed that the inverse association between a typical Tuscan dietary pattern and CES-D scores was similar in participants with major depressive symptoms compared with the observed association of our main analyses, thus confirming our results that a typical Tuscan diet is associated with mild as well as major depressive symptoms.
In addition, the population characteristics and different cultures are not always comparable. It is assumed that not only the quality of the dietary pattern is essential but also the context where people consume their meals might be influential. For example, in the Tuscan population, consuming meals together with friends or family and taking time for meals are considered important. These contextual aspects may be part and parcel of the typical Tuscan dietary pattern, which might reflect a higher quality of life in those consuming it. Overall, future studies should evaluate the role of the social aspects of meal consumption and dietary patterns in relation to depressive symptoms.
Conclusion
In summary, our results indicate that a typical Tuscan dietary pattern with high intakes of vegetables, olive oil, fish, fruits, cereals and moderate intakes of wine and red and processed meat is consistently associated with lower depressive symptoms over a 9-year period in Italian adults. Our results suggest that diet could be a plausible tool to test in practice to lower depressive symptoms.
Acknowledgements
The authors thank the participants of the InCHIANTI study for their continuing contributions, all participating departments in the InCHIANTI team and Wim Busschers and Moniek de Goeij for their contribution to the data analysis.
Funding for this research was provided by EU FP7 MooDFOOD Project ‘Multi-country cOllaborative project on the rOle of Diet, FOod-related behaviour, and Obesity in the prevention of Depression’, grant agreement no. 613598. The InCHIANTI study baseline (1998–2000) was funded by the Italian Ministry of Health (ICS110.1/RF97.71) and in part by the US National Institute on Aging, Bethesda, MD (contracts: 236 MD 916413 and 236 MD 821336). The InCHIANTI follow-up 1 (2001–2003) was funded by the US National Institute on Aging (contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI follow-ups two and three (2004–2010) were financed by the US National Institute on Aging (contract: N01-AG-5-0002), supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
The authors’ contributions are as follows: S. B. and L. F. designed the research and had full access to all the data in the study; E. V., K. S. and M. N. developed the research questions; E. V. analysed the data and had primary responsibility for the final content of the manuscript; E. V. and M. N. drafted the manuscript. All authors critically reviewed the manuscript for important intellectual content and approved the final version of the manuscript. The analyses of the present study were entirely independent, with no industry association.
None of the authors has any conflicts of interest to declare.








