Flow-diverting stents (FDSs) were approved for the treatment of aneurysms of the intracranial internal carotid arteries. Given their advantages that allow for the occlusion of aneurysms not amenable to coiling, including fusiform, blister, and dissecting aneurysms, they are increasingly being used to treat ruptured intracranial aneurysms within the posterior circulation. Reference Griessenauer, Ogilvy and Adeeb1
A recently synthesized meta-analysis showed that FDS demonstrate higher rates of morbidity and mortality when used in ruptured posterior circulation aneurysms (27%) compared with ruptured anterior circulation aneurysms (12%). Reference Cagnazzo, Di Carlo, Cappucci, Lefevre, Costalat and Perrini2 However, given the exceedingly high mortality of untreated ruptured intracranial aneurysms, Reference Korja, Kivisaari, Rezai Jahromi and Lehto3 the relatively increased complication rates of posterior circulation flow diversion may be acceptable.
The technology involved in the manufacture of FDS continues to advance with an expanding array of new devices. A recent example is the Pipeline Flex Embolization Device with Shield Technology (PED-Shield, Medtronic Neurovascular, Irvine, California, USA), which includes surface modification with phosphorylcholine coating to render is device less thrombogenic. A few recent publications have shown the safety of PED-Shield for endovascular repair of ruptured aneurysms with single antiplatelet therapy (SAPT). Reference Manning, Cheung, Phillips and Wenderoth4,Reference Bender, Zarrin and Jiang5
This report describes the use of the PED-Shield for the treatment of a ruptured dissecting posterior circulation aneurysm with SAPT complicated with delayed in-stent thrombosis. A female patient in her 40s developed a severe thunderclap headache at home. The next morning, she was found collapsed at home, confused, and incontinent. She was quickly transported to hospital.
In the emergency department, she was fully alert and responsive but reported horizontal diplopia and a severe (10/10) headache. Examination revealed a left sixth nerve palsy. World Federation of Neurosurgical Societies grading was 1. Her past medical history included dyslipidemia, migraine headaches, and a remote smoking history of 10 pack-years.
Cranial CT scan demonstrated diffuse subarachnoid haemorrhage (SAH) without intraventricular extension, consistent with Modified Fisher grade 3. Cranial CT angiography identified a dissecting aneurysm involving the V4 segment of the left vertebral artery (VA), subsequently confirmed on cerebral angiography. External ventricular drain was not required.
After a loading dose of acetylsalicylic acid (ASA) 325 mg and under general anesthesia, endovascular repair of the left V4 dissecting pseudoaneurysm was performed on post-SAH day 2, using overlapping PED-Shield devices. A triaxial system was utilized, and with full heparinization, a Phenom 27 (Medtronic Inc) was advanced over a 0.014 inch microguidewire into the left VA, beyond the abnormal vessel segment, and into the proximal basilar artery. Two overlapping PED-Shield devices (4.75 × 14 mm and 4.5 × 16 mm) were successfully deployed within the distal V4 segment beyond the origin of the left posterior inferior cerebellar artery (PICA) (Figure 1). The second PED-Shield was used because aneurysm filling remained unchanged after deployment of the first. The time interval between ASA administration and first PED-Shield deployment was 5 h.
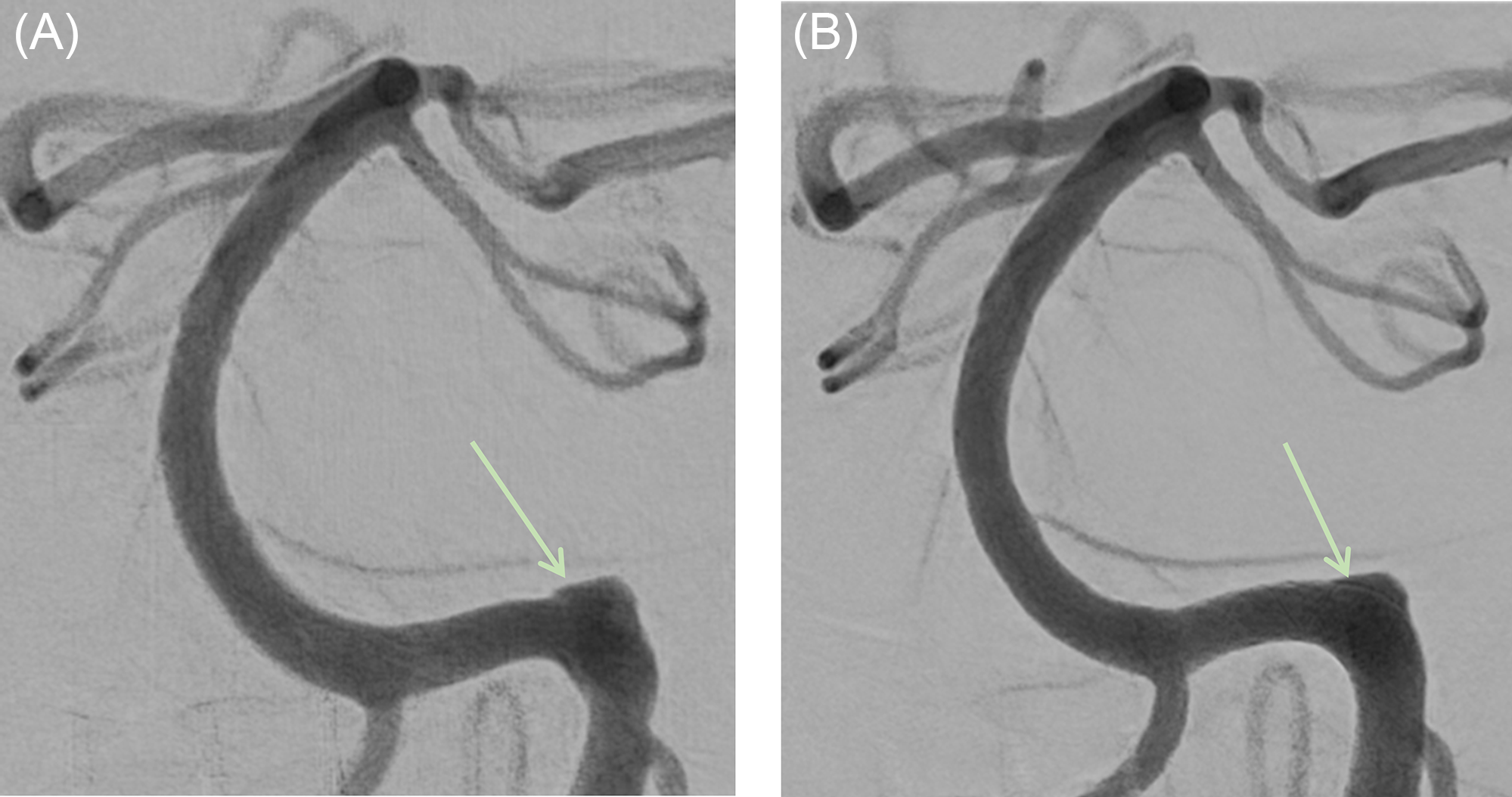
Figure 1: Digital subtraction angiogram of the posterior circulation showing the aneurysm (green arrows). (A) A magnified image of the distal left vertebral artery and the basilar artery in frontal projection prior to flow-diverting stent (FDS) placement. (B) The same view after FDS placement.
Repeat angiographic runs confirmed patency of the left VA, basilar artery, and associated branches. Reflux opacification was seen in the right VA. Decreased filling was observed in the aneurysm post second PED-Shield implantation (O’Kelly-Marotta scale B2). The patient recovered well and remained on SAPT (ASA 81 mg daily) and eventually discharged home on day 14 post-SAH with persistent double vision due to a partial left sixth nerve palsy.
The patient presented again to hospital on day 15 post-SAH (day 13 post-procedure), with sudden onset headache and left-sided neck pain with mild incoordination. Neurological examination demonstrated dysmetria of the left-upper and -lower extremity and a persistent left sixth nerve palsy.
CT and MR angiography showed acute thrombosis of the left extracranial V3 segment extending to the intracranial left V4 segment just proximal to the vertebrobasilar junction, with complete thrombosis of the PED-Shield stents (Figure 2A,B). The contralateral right VA and basilar artery remained patent. There was no opacification of the left PICA. MRI demonstrated small ischemic infarcts within the left cerebellar hemisphere and in the bilateral occipital lobes, likely secondary to the in-stent thrombosis and left PICA and VA occlusion (Figure 2C,D). The patient was monitored for 24 h then discharged home in stable condition. She was seen in clinic after 30 d, at which point the sixth nerve palsy and left extremity symptoms had improved. She resumed function at baseline and returned to work.

Figure 2: Panels A and B represent CT angiogram images at the level of the stent (green arrows). (A) During the index hospital admission, on postoperative day 7, demonstrating stent patency. (B) On readmission, postoperative day 13, demonstrating stent occlusion. Panels C and D represent Two MRI axial slices demonstrating areas of diffusion restriction representing infarcts (yellow arrows).
The PED-Shield device with surface modification was designed to be less thrombogenic, and early reports have demonstrated that it can be safely used to treat ruptured intracranial aneurysms with SAPT. Reference Hanel, Aguilar-Salinas, Brasiliense and Sauvageau6 The main advantage of SAPT over dual antiplatelet therapy (DAPT), and the rationale for this case, is the theoretical reduction in bleeding risk in cases of ruptured aneurysms. However, prior to the PED-Shield device, FDSs were predominantly used with DAPT.
A literature review revealed a recent study of 50 patients treated using the PED-Shield with DAPT, in which only a single case of in-stent thrombosis occurred in a distal internal carotid artery and none in VA aneurysms. Reference Martinez-Galdamez, Lamin and Lagios7 Another recent study of 14 patients with ruptured aneurysms (3 in VAs) treated using PED-Shield demonstrated no cases of in-stent thrombosis with SAPT, with some patients receiving more than one PED-Shield (average 1.2). Reference Manning, Cheung, Phillips and Wenderoth4
Ischemic stroke in the territory of a treated vessel is a known complication of flow diverter use. A multicenter retrospective study found that ischemic stroke occurred in 22.5% of 129 patients who had PED (without Shield technology) placement in the posterior circulation, although many were considered minor. Reference Griessenauer, Ogilvy and Adeeb1 Another study showed that posterior circulation ischemic stroke with clinical symptoms occurred in 7.3% of patients (n = 55). Reference Kallmes, Hanel and Lopes8
Most patients in these studies were treated with a single stent, whereas our patient had two overlapping stents. This may have been a significant factor that led to the delayed in-stent thrombosis. A similar outcome was reported for a patient with a ruptured posterior circulation aneurysm who received SAPT and two PED-Shield devices. Reference Hanel, Aguilar-Salinas, Brasiliense and Sauvageau6
Considering this case and the literature review findings, DAPT may be the safer approach, particularly in the early postoperative period. Although PED-Shield was designed to be less thrombogenic, more data are required to consider its use with SAPT, and this is especially true when multiple overlapping stents are used.
Acknowledgments
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures
Dr. Kaka has nothing to disclose.
Dr. van Adel has nothing to disclose.
Dr. Larrazabal has nothing to disclose.
Informed consent was obtained from the patient for this case report.
Statement of Authorship
HK contributed to writing this article, obtained consent from the patient, performed the relevant literature search, and image retrieval from PACS.
RL was the primary operator during the procedure and made technical and clinical treatment decisions, as well as follows up clinical appointments.
BVA contributed to article writing and the literature search and assisted in image retrieval. He also assessed the patient during their second hospital presentation.




