Ragweed parthenium (family: Asteraceae) is one of the most important invasive weed species now found in more than 40 countries around the world, while North and South America are part of its native range (Bajwa et al. Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016). It is a warm-season annual weed but sometimes also behaves like a short-lived perennial (Adkins and Shabbir Reference Adkins and Shabbir2014). Characterized by an erect stem and deep taproot, it can grow up to 2.0 m tall under good moisture and soil conditions. This weed causes severe losses to crop and pasture productivity, native biodiversity, and the livestock sector in its introduced range (Adkins and Shabbir Reference Adkins and Shabbir2014). It also causes severe health problems, including contact dermatitis, asthma, pollen allergy, and breathing issues, in human beings and animals (Navie Reference Navie2002). Ragweed parthenium is one of the “Weeds of National Significance” in Australia due to its serious negative impacts on pastures, crop production, and the beef industry (Adkins and Shabbir Reference Adkins and Shabbir2014). Therefore, a better understanding of its invasive biology is crucial to predict its future spread and to devise suitable management strategies in Australia and elsewhere.
Ragweed parthenium has two biotypes in Australia that are differentiated on the basis of their introduction, colonization ability, and demographic spread (Bajwa et al. Reference Bajwa, Chauhan and Adkins2017). The first introduction occurred around the mid-1940s in Toogoolawah (north of Brisbane, Queensland). That population has never spread more than 10 km from its point of origin and is now known as the Toogoolawah biotype. The second introduction occurred in 1958 in Clermont (central Queensland); after a short establishment period, this biotype started to spread rapidly in the mid-1970s, and by 2003 it covered more than 520,522 km2. This biotype is invasive and is known as the Clermont biotype. It is believed that the Toogoolawah and Clermont biotypes were introduced independently from a northern and a southern Texas race, respectively (Adkins and Shabbir Reference Adkins and Shabbir2014). The two biotypes have shown significant differences in their genetic makeup, morphological attributes, and adaptive ability (Bajwa et al. Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016, Reference Bajwa, Chauhan and Adkins2017; Hanif et al. Reference Hanif, Adkins, Prentis, Navie and O’Donnell2012; Navie Reference Navie2002). Therefore, these biotypes of ragweed parthenium represent a perfect model for comparative studies into the invasion biology of this noxious weed.
In a global perspective, ragweed parthenium has been recognized as a highly invasive weed species. Several factors have been associated with high invasiveness of ragweed parthenium, including high seed production, a persistent soil seedbank, a competitive and fast growing habit, biological life-cycle plasticity, allelopathic capacity, and abiotic stress tolerance (Adkins and Shabbir Reference Adkins and Shabbir2014; Bajwa et al. Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016, Reference Bajwa, Chauhan and Adkins2017; Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a, Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017b). However, the information on seed germination ecology of this species, especially considering the two distinct biotypes, is limited. Germination ecology has a key role in the establishment of weeds in any agroecosystem (Bajwa et al. Reference Bajwa, Mahajan and Chauhan2015; Chauhan and Johnson Reference Chauhan and Johnson2010), because it affects the competitive ability of weeds and the management options chosen. Ragweed parthenium’s success under a diverse range of climatic conditions has been attributed to its rapid, strong germination ability followed by vigorous early plant growth (Bajwa et al. Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016, Reference Bajwa, Chauhan and Adkins2017).
More frequent droughts with rising temperatures and atmospheric carbon dioxide concentrations are predicted in the future for eastern Australia (Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a). In response to such climatic changes, the biology of many alien invasive weed species is expected to change in a way that will better aid their further invasion (Bajwa et al. Reference Bajwa, Chauhan and Adkins2017; Nguyen et al. Reference Nguyen, Bajwa, Navie, O’Donnell and Adkins2017a). In the meantime, salinity has become a significant problem in many parts of Australia, with about 60% of the total 20 million ha of cropping land having sodic soils (Rengasamy Reference Rengasamy2010). Due to high accumulation of alkaline salts, the pH of many salt-affected soils has also increased, with some as high as 9.0 (de Caritat et al. Reference de Caritat, Cooper and Wilford2011). The germination and early seedling growth of any invasive species in response to these factors may determine their invasive potential and pattern of spread in the future. These factors will also affect the efficacy of the existing management approaches used. Therefore, it is important to study the germination ecology of this species in relation to the above-mentioned environmental factors. Earlier studies have shown that ragweed parthenium can germinate over a wide range of temperatures and moisture levels (Navie Reference Navie2002; Pandey and Dubey Reference Pandey and Dubey1988; Tamado et al. Reference Tamado, Schutz and Milberg2002; William and Groves Reference Williams and Groves1980). However, no study to date has looked at all the major environmental stresses over a wide range of levels while taking into account biotype differences. Thus, existing knowledge is not comprehensive enough to describe the germination ecology of this species.
This study was undertaken (1) to evaluate the germination response of ragweed parthenium under a range of constant and alternating day/night temperature and light conditions and moisture, salt, and pH levels applied under a controlled environment; and (2) to investigate whether the two biotypes of this weed with contrasting invasive ability and invasion history differ in their germination ecology. The germination response to complete darkness (24 h) would indicate whether seedlings of this weed species could emerge from seeds buried at soil depths or underneath crop residues. This information could then be used to select an appropriate tillage system (e.g., no-till or conventional tillage systems). The constant day/night temperature treatment would enable identification of the base temperature for germination of this species. This treatment might also allow for the identification of the minimum and maximum temperatures below and above which the seeds cannot germinate. It is important to note that seeds placed on or near the soil surface experience fluctuating temperatures, but the fluctuation range decreases with an increase in burial depth under field conditions. Therefore, it is important to investigate the germination response under constant as well as fluctuating day/night temperatures.
Materials and Methods
Seed Collection and Storage
Fresh achenes (hereafter referred to as “seeds”) of Clermont and Toogoolawah biotypes of ragweed parthenium were collected from several plants of each biotype raised in a controlled environment greenhouse at the University of Queensland, Gatton Campus (−27.554°S, 152.345°E), Queensland, Australia, during December and January 2015–16. Seeds of both biotypes were collected earlier from the field and then used to raise seed-bearing plants in the greenhouse under identical conditions. The seeds were hand harvested when they had reached physiological maturity (80 d after sowing), placed in labeled paper bags, and kept under laboratory conditions (22 C). After seeds had reached a constant moisture content for 1 wk, they were separated from any remaining flower debris. Filled (dark-colored) single-seeded fruit (hereafter referred to as “seeds”) were separated from unfilled (light-colored) seeds; placed in labeled, airtight glass bottles; and stored in the dark at 20 C until needed. As the seeds used in this study came from plants grown under identical conditions and then stored under identical conditions, the effect of the maternal environment was kept to a minimum and any germination differences seen in subsequent germination tests can be attributed to differences in biotype.
General Seed Germination Test Protocol
All the experiments involving alternating day/night temperatures were undertaken in germination incubators (TRIL-750 Illuminated Refrigerator Incubator, Thermoline, Wetherill Park, Australia) with a cool white fluorescent light that produced a photosynthetic photon flux density (PPFD) of 100 µmol m−2 s−1. Seeds were surface sterilized by being shaken in sodium hypochlorite (1% v/v) for 1 min and then rinsed several times in sterilized, reverse-osmosis water (RO water) before the start of each germination trial to avoid fungal growth on seeds. Germination tests were performed by placing 25 seeds evenly across the surface of 9-cm-diameter petri dishes each containing a double layer of Whatman No. 1 filter paper (Navie Reference Navie2002; Tamado et al. Reference Tamado, Schutz and Milberg2002). Filter papers were then moistened with 5 ml sterilized RO water or a treatment solution, and an extra amount of the appropriate solution was added when required. petri dishes were placed on a thick layer of moistened paper towel in a transparent plastic box with an airtight lid to reduce evaporation from the dishes. On the thermogradient bar (see “Effects of Temperature and Light”), petri dishes were wrapped with transparent parafilm to reduce evaporation.
The experiments to determine the effect of osmotic stress, salt stress, and pH on germination were conducted in an incubator configured for 25/15 C day/night thermoperiod, with a 12-h photoperiod matched with the time of the thermoperiod. These conditions were found to be optimum from the first experiment evaluating the effect of light and temperature on seed germination. To simulate complete dark conditions, petri dishes were wrapped in three layers of aluminum foil and only opened once for a germination count after 21 d. In case of the 12-h photoperiod, petri dishes were not covered by aluminum foil and the counting of the number of germinated seeds started on the third day of incubation and continued up to 21 d after incubation. Seeds were considered to have germinated when the radicle was at least 2-mm long. Given that the germination of both seed lots reached 100% in RO water controls in at least one treatment, the seed lots were considered to be 100% viable, and any germination reduction from this value could be attributed to the respective treatments. Seed germination percentages were calculated for each replicate based on the aggregated germination count after 21 d.
Effects of Temperature and Light
To determine the effect of constant temperature and light, seeds of both biotypes were incubated in a thermogradient bar (Thermoline). This bar consisted of 10 equal-sized chambers, each with a different but constant temperature and illuminated by overhead cool white fluorescent light producing a daytime PPFD of 100 µmol m−2 s−1 at the level of petri dish incubation. The temperatures in the 10 chambers were monitored regularly and ranged from a low of 8 C to a high of 36 C (i.e., 8, 11, 14, 17, 20, 23, 26, 29, 32, and 36 C). To determine the effects of alternating day/night temperature and day length, seeds of both biotypes were incubated at five different alternating temperatures (15/5, 20/5, 25/15, 30/20, and 35/25 C) under two different photoperiods: complete darkness (24-h dark) or a 12-h photoperiod. Other conditions were the same as described for the standard germination test.
Effect of Osmotic Stress
To examine the effect of osmotic stress on seed germination, aqueous solutions of osmotic potentials of −0.1, −0.2, −0.4, −0.6, −0.8, −1.0, and −1.2 MPa were prepared with polyethylene glycol 8000 (Sigma-Aldrich, St Louis, MO) as described by Michel (Reference Michel1983). These levels of osmotic potential were selected based on previous studies on ragweed parthenium and other weed species (Chauhan et al. Reference Chauhan, Gill and Preston2006; Chauhan and Johnson Reference Chauhan and Johnson2008; Tamado et al. Reference Tamado, Schutz and Milberg2002) and are similar to those found in different parts of Australia where this weed may invade. An RO water treatment was used as a control. The experiment was conducted in an incubator set at a 25/15 C day/night thermoperiod with a 12-h photoperiod matched to the thermoperiod. Other conditions were the same as described in the standard germination test.
Effect of Salt Stress
The effect of salinity on seed germination was determined by using NaCl solutions of 0, 25, 50, 100, 150, 200, and 250 mM. This range represents soil salinity levels found in different parts of Australia where this weed may invade (Rengasamy Reference Rengasamy2006, Reference Rengasamy2010). An RO water treatment was maintained as a control. The experiment was conducted in an incubator set at a 25/15 C day/night thermoperiod with a 12-h photoperiod matched to the thermoperiod. Other conditions were the same as described in the general germination test.
Effect of pH
The pH levels were selected based on the literature suggesting that pH of Australian soils varies between 4.0 and 10.0 (de Caritat et al. Reference de Caritat, Cooper and Wilford2011) and represent soil pH levels found in different parts of Australia where this weed may invade. Buffer solutions with pH values of 4.0 to 10.0 were prepared according to the method described by Chachalis and Reddy (Reference Chachalis and Reddy2000). A 2 mM solution of MES [2-(N-morpholino)ethanesulfonic acid] was adjusted with 0.1 N hydrogen chloride (HCl) or sodium hydroxide (NaOH) to obtain the solutions of pH 4.0, 5.0, and 6.0. A 2 mM solution of HEPES [N-(2-hydroxymethyl)piperaziine–N’–(2-ethanesulfonic acid)] was adjusted with 0.1 N NaOH obtain the solutions of pH 7.0 and 8.0. Buffer solutions of pH 9.0 and 10.0 were prepared with 2 mM tricine [N-Tris (hydroxymethyl)methylglycine] and adjusted with 0.1 N NaOH. Unbuffered RO water (pH 6.4) was used as a control. Other conditions were the same as described in the general germination test.
Statistical Analyses
All the experiments were arranged in a completely randomized design giving equal importance to all the treatment factors. Each experiment was conducted two times with three replicates of 25 seeds. The significance of means of individual treatment factors and their interactions was estimated through ANOVA in all experiments. The homogeneity of variance was not improved by square-root or logarithmic data transformation; ANOVA was therefore performed on the nontransformed percent germination values (STATISTIX v. 8.1 statistical software). Data from the repeated experiments were subjected to ANOVA, and there were no significant time by treatment interactions (P<0.05); data were therefore pooled for analysis. Means were separated using Fisher’s protected LSD test at P=0.05. Curve fitting was not appropriate for the temperature and light and pH experimental data. Thus, the treatment means were presented in bar charts with ±SE of means using SigmaPlot v. 13. Germination percentages at different osmotic potentials or NaCl concentrations were fit to a two-parameter simple linear model using SigmaPlot. The model fitted was
where y is germination percentage at osmotic potential of x MPa or NaCl concentration of x mM.
The R2 values were used to determine the goodness of fit to all selected models.
Results and Discussion
Effects of Light and Temperature
A significant interaction among illumination conditions, temperature levels, and ragweed parthenium biotypes was observed in the incubator experiment (alternating day/night temperatures used) and in the thermogradient bar experiment (constant day/night temperatures used). Overall, the Clermont biotype exhibited a higher germination percentage after 21 d of incubation compared with the Toogoolawah biotype, and this was seen across all light and temperature treatments (Figures 1 and 2). Similarly, germination percentage was higher for both biotypes under a 12-h photoperiod compared with complete darkness when studied at both constant and alternating day/night temperatures (Figures 1 and 2). Maximum germination percent (100%) was observed for the Clermont biotype under a 12-h photoperiod coupled with constant day/night temperatures of 14 to 23 C (Figure 1; this was the same for 24 h). The Toogoolawah biotype obtained maximum germination percentage (97%) under a 12-h photoperiod at the constant temperature of 23 C (Figure 1). The Clermont biotype was able to germinate over a wider range of constant day/night temperatures (8 to 35 C); however, it showed 36% less germination under complete darkness as compared with what was produced under a 12-h photoperiod, and there was no germination at a constant 35 C under complete darkness (Figure 1). Although the Toogoolawah biotype also germinated over the range of 8 to 35 C under a 12-h photoperiod, there was no germination under complete darkness at the low (8 C) or high (29 to 35 C) constant temperatures. At alternating day/night temperatures, both biotypes were able to germinate over a wide range of thermoperiods (15/5 to 35/25 C) under a 12-h photoperiod or complete darkness (Figure 2). Maximum germination (100%) was observed for the Clermont biotype under a 12-h photoperiod with a matched thermoperiod of 25/15 C (Figure 1). Minimum germination was observed for the Toogoolawah biotype under complete darkness at a 12-h thermoperiod of 35/25 C.
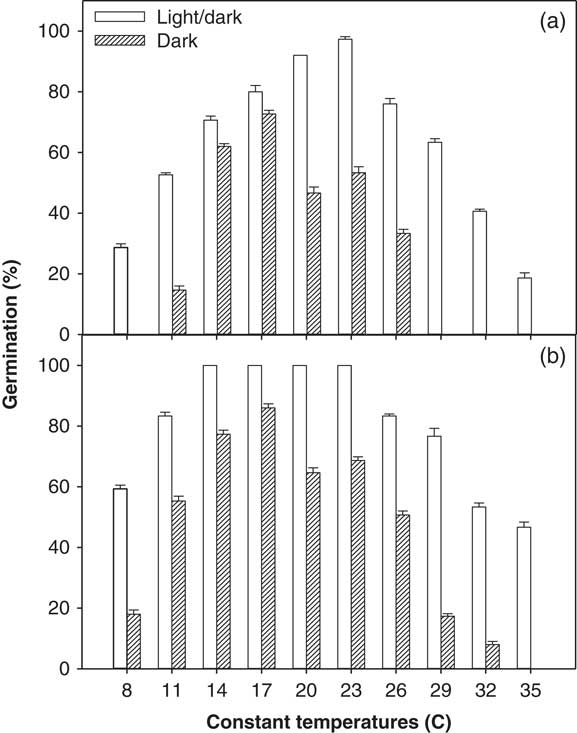
Figure 1 Effect of constant day/night temperatures (8 to 35 C) on the germination of (a) Toogoolawah and (b) Clermont biotypes of ragweed parthenium under light/dark (12-h photoperiod) and complete dark (24-h photoperiod) regimes. Seeds were incubated for 21 d. Data represent the mean±SE of the mean (n=6).
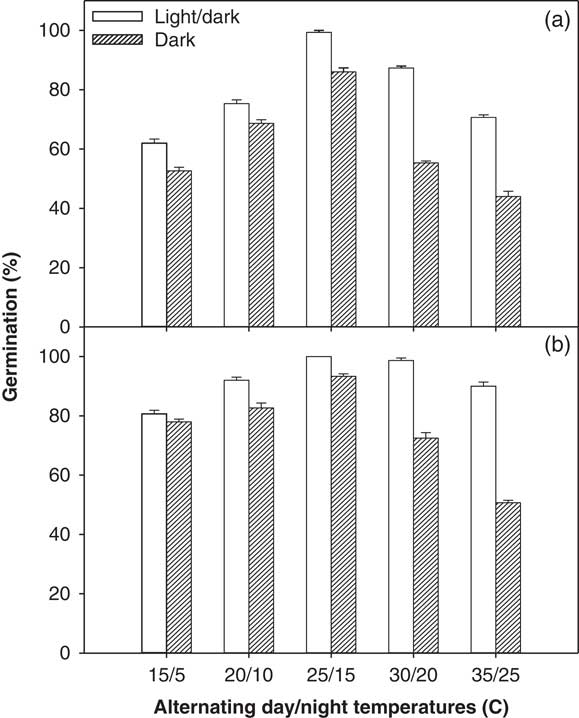
Figure 2 Effect of alternating day/night temperatures (15/5 to 35/25 C) on the germination of (a) Toogoolawah and (b) Clermont biotypes of ragweed parthenium under light/dark (12-h photoperiod) and complete dark (24-h photoperiod) regimes. Seeds were incubated for 21 d. Data represent the mean±SE of the mean (n=6).
The higher germination of both biotypes under a fluctuating light/dark condition compared with complete darkness across the temperature regimes is consistent with the fact that ragweed parthenium germination has been reported to be triggered by light, possibly due to release from dormancy (Navie Reference Navie2002). An Ethiopian ragweed parthenium population also showed 37% to 43% less germination under complete darkness compared with a 12-h photoperiod (Tamado et al. Reference Tamado, Schutz and Milberg2002). Moreover, freshly collected seeds were unable to germinate under complete darkness, and it was proposed that this was due to innate dormancy that could not be rapidly overcome in the absence of light (Tamado et al. Reference Tamado, Schutz and Milberg2002). Interestingly, seeds stored for a longer period could germinate when imbibed under complete darkness. This is consistent with the view that germination can take place in darkness but only after the seeds have had time to lose dormancy, whereas dormancy can be lost much more rapidly in seeds imbibed in the light. William and Groves (Reference Williams and Groves1980) reported that ragweed parthenium had high germination under complete darkness at different alternating day/night temperatures, but the storage time of the seed was not reported. In contrast, our results showed that germination was reduced in complete darkness compared with a 12-h photoperiod at alternating day/night temperatures. In the study by William and Groves (Reference Williams and Groves1980), petri dishes were opened at intervals for short durations to undertake germination counts, and it is likely that such short-term exposures might have facilitated the seeds overcoming dormancy (Navie Reference Navie2002; Tamado et al. Reference Tamado, Schutz and Milberg2002). Pandey and Dubey (Reference Pandey and Dubey1988) reported a significant increase in ragweed parthenium germination under complete darkness after a light pretreatment. In the present study, we also observed a substantial increase in the final germination percentage (up to 40%) and germination rate when petri dishes from complete darkness were shifted to a 12-h photoperiod after 21 d (unpublished data). Karlsson et al. (Reference Karlsson, Tamado and Milberg2008) also reported that ragweed parthenium germinated more under light compared with continuous dark conditions. It was suggested that light may help this species to break dormancy and may also improve the rate of germination (Karlsson et al. Reference Karlsson, Tamado and Milberg2008).
The germination ability of ragweed parthenium over a wide range of alternating day/night temperatures has been reported in earlier studies (Navie Reference Navie2002; Pandey and Dubey Reference Pandey and Dubey1988; Tamado et al. Reference Tamado, Schutz and Milberg2002; William and Groves Reference Williams and Groves1980). However, these studies did not compare two different biotypes at alternating day/night temperatures as was done in the present study. William and Groves (Reference Williams and Groves1980) reported that ragweed parthenium germination was significantly reduced at temperatures below 5 C and above 35 C. In contrast, Tamado et al. (Reference Tamado, Schutz and Milberg2002) reported no reduction in germination at an alternating day/night temperature of 35/25 C compared with 25/15 C. In support of Williams and Groves (Reference Williams and Groves1980), we found a 27% and a 41% reduction in germination at 35/25 C and an 18% and 41% reduction at 15/5 C for both Clermont and Toogoolawah biotypes, respectively, when compared with the germination levels achieved at the optimal temperature of 25/15 C (Figure 2). Significantly higher germination of the Clermont biotype compared with the Toogoolawah biotype is consistent with the greater invasiveness of the former biotype (Bajwa et al. Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016). Overall, the germination ability of these two ragweed parthenium biotypes over the wide range of temperatures may enable them to invade farther into the Australian landscape. Moreover, a significantly higher germination, especially of the Clermont biotype (>80%), at relatively higher day/night temperatures (35/25 C) may favor its spread in future under climate change.
Effect of Osmotic Stress
A strong linear negative relationship was observed between the osmotic potential of the imbibing solution and germination percentage for both biotypes (Figure 3) down to −1.00 MPa. However, no germination was observed beyond this point. The interaction of biotype and osmotic potential level was also significant (P<0.05). The Clermont biotype showed a 20% higher germination percentage compared with the Toogoolawah biotype across all the osmotic potential treatments used (P<0.05). Greater than 50% of the seeds of the Clermont biotype germinated at −0.60 MPa, whereas the germination of the Toogoolawah biotype was reduced to 36% at this level (Figure 3). A 50% reduction in germination of the Toogoolawah and Clermont biotypes was observed at the osmotic potentials of −0.48 and −0.60 MPa, respectively.
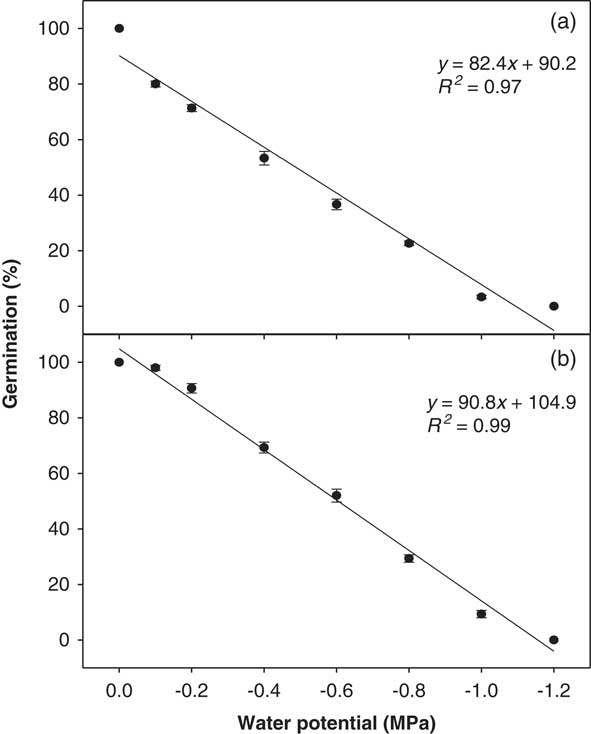
Figure 3 Effect of osmotic potential on the germination of (a) Toogoolawah and (b) Clermont biotypes of ragweed parthenium at alternating day/night temperatures of 25/15 C under a 12-h photoperiod. Seeds were incubated for 21 d. The bold trend lines represent the linear regression model (y=a+bx) fit to the data. Vertical bars represent ±SE of the mean (n=6).
These results indicate that ragweed parthenium has an exceptional ability to germinate at relatively low levels of soil moisture. It is also clear that the Clermont biotype can better germinate under such conditions compared with the Toogoolawah biotype, which is consistent with high invasiveness and drought tolerance of the Clermont biotype during its life cycle (Bajwa et al. Reference Bajwa, Chauhan, Farooq, Shabbir and Adkins2016, Reference Bajwa, Chauhan and Adkins2017). Tamado et al. (Reference Tamado, Schutz and Milberg2002) reported a complete germination inhibition beyond −0.52 MPa osmotic potential at the mean day/night temperature of 27 C. However, the same study reported that there was about 20% germination even at −0.86 MPa at a lower mean day/night temperature of 20 C, which is in agreement with our results. William and Groves (Reference Williams and Groves1980) reported a 50% germination of ragweed parthenium seeds at −0.70 MPa under natural soil conditions. The germination was 91% at field capacity (control) in that study and was completely inhibited at −0.90 MPa. These variations might be associated with temperature and other germination conditions. Unlike many other weed species, ragweed parthenium can germinate at a relatively very low substrate moisture availability. For instance, the germination of two Asteraceae weed species, coat buttons (Tridax procumbens L.) and siamweed [Chromolaena odorata (L.) King & H. E. Robins.], was completely inhibited at −0.80 and −1.00 MPa osmotic potentials, respectively (Chauhan and Johnson Reference Chauhan and Johnson2008). Chauhan et al. (Reference Chauhan, Gill and Preston2006) reported up to a 90% inhibition in the germination of annual sowthistle (Sonchus oleraceus L.) at −0.60 MPa osmotic potential. Thus, the invasive Clermont biotype of ragweed parthenium has a great potential to expand its invasion into the more arid regions of Australia, and this is already being observed. However, the seedling, then plant growth response under such conditions, now needs to be studied to determine the full invasive potential of this weed in these arid regions.
Effect of Salt Stress
A strong linear negative relationship was also observed between NaCl concentration and ragweed parthenium germination percentage (Figure 4). The interaction of biotypes and osmotic potential levels was significant (P<0.05). Highest germination of the Clermont (100%) and the Toogoolawah (96%) biotypes were observed in the control. Both biotypes were able to germinate at a very high NaCl concentration of 250 mM. The germination of the Toogoolawah biotype was more affected by salt stress than the Clermont biotype. A 50% reduction in the germination of the Toogoolawah and Clermont biotypes was caused by 99 and 154 mM NaCl, respectively (Figure 4).
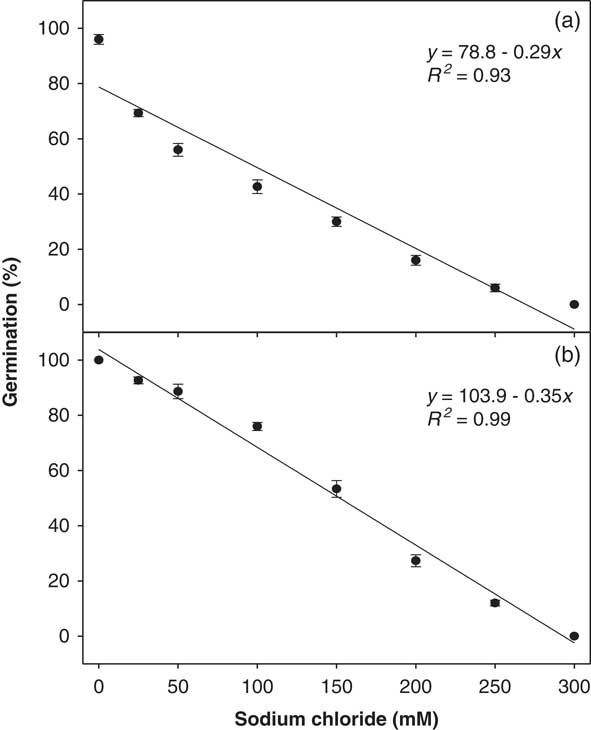
Figure 4 Effect of sodium chloride concentrations on the germination of (a) Toogoolawah and (b) Clermont biotypes of ragweed parthenium at alternating day/night temperatures of 25/15 C under a 12-h photoperiod. Seeds were incubated for 21 d. The bold trend lines represent the linear regression model (y=a+bx) fit to the data. Vertical bars represent ±SE of the mean (n=6).
It is thus clear that ragweed parthenium also has the ability to germinate under relatively high salt concentrations, and the Clermont biotype is able to tolerate such conditions better than the Toogoolawah biotype. The reduction or inhibition of germination under salt stress is thought to be due to various physiological disruptions in metabolism caused by ion toxicity or high osmotic stress (Farooq et al. Reference Farooq, Hussain, Wakeel and Siddique2015). Khurshid et al. (Reference Khurshid, Nasim, Bajwa and Adkins2012) have also reported a substantial reduction in seed germination and early growth of ragweed parthenium under saline conditions. However, no specific details were provided on the length of the germination study or the temperature conditions used. Given the increasing problem of salinity in arid environments of Australia, ragweed parthenium could become more of a problem in such areas in the future. Although ragweed parthenium can germinate at relatively high salt concentrations, it will be interesting to study its growth and reproductive responses under such conditions to better predict its future invasion pattern in these salt-affected landscapes.
Effect of pH
The germination of ragweed parthenium was slightly affected by pH (4.0 to 10.0; Figure 5). A significant interaction between biotypes and pH levels was observed (P<0.05). There was no statistical difference for the germination of the Clermont biotype across the eight different levels of pH studied; the seed germination was 100% at pH levels 5.0 to 9.0 (Figure 5). However, the Toogoolawah biotype had its highest germination of 94% with the RO water control and was reduced by 18% and 13% at pH 4.0 and 5.0, respectively. On the other hand, the lowest germination percentage (69%) for this biotype was obtained at a highly alkaline solution of pH 10.0. Overall, the Clermont biotype had an 18% higher germination percentage compared with the Toogoolawah biotype across different pH levels.
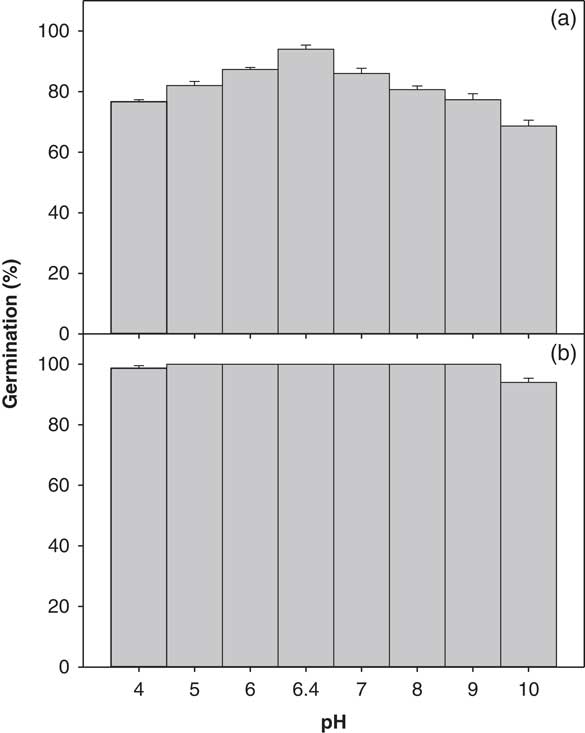
Figure 5 Effect of pH on the germination of (a) Toogoolawah and (b) Clermont biotypes of ragweed parthenium at alternating day/night temperatures of 25/15 C under a 12-h photoperiod. Seeds were incubated for 21 d. Data represent the mean±SE of the mean (n=6).
These results show that ragweed parthenium in general, and specifically its invasive biotype Clermont, was not affected by a wide range of pH levels. In fact, these pH regimes represent most of the soil types in Australia (Rengasamy Reference Rengasamy2006). Therefore, pH is not seen as a limiting factor for ragweed parthenium germination. This attribute is common to some other important Asteraceae weed species such as annual sowthistle (Chauhan et al. Reference Chauhan, Gill and Preston2006).
In conclusion, ragweed parthenium can germinate over a wide range of environmental conditions. The invasive and more commonly found biotype, Clermont, had higher germination compared with the noninvasive biotype, Toogoolawah, under normal and stress conditions. It appears that ragweed parthenium can germinate without light, but a 12-h photoperiod was ideal for its maximum germination. Thus, it may be possible to reduce germination of this species in cropping systems by promoting deep tillage or residue management practices that will help bury seeds to a greater depth and limit their exposure to light. This species has the ability to germinate over a wide range of constant (8 to 35 C) and alternating (15/5 to 35/25 C) temperatures, which could be the reason for its year-round existence in many parts of the world. The ability to germinate at very high osmotic and salt stresses and over a wide range of pH levels may be a contributing factor in the high invasiveness of ragweed parthenium. It is important to consider these thresholds while planning any improved management strategies. These results could serve as baseline information for predictive modeling for ragweed parthenium’s future invasive spread in Australia and in other countries with similar climatic conditions. This study also provides pragmatic implications for management of this problematic weed species. Further studies should be conducted to evaluate the effect of environmental conditions on ragweed parthenium growth and reproductive potential to better understand its invasion biology and develop suitable management strategies.
Acknowledgments
AAB is grateful to the Australian Government and the University of Queensland, Australia, for the provision of a Research Training Program Scholarship and UQ Centennial Scholarship, respectively. The authors also wish to thank Rana Nadeem Abbas and Katherine Raymont for their assistance in conducting the experiments.







