Introduction
Digenetic trematodes are parasitic platyhelminths with a complex life cycle that involves one or more intermediate host, and vertebrates as definitive hosts for successful completion. Molluscs serve as first intermediate hosts and their distribution accounts for the occurrence of trematode species in a particular area. They are infected by the free-living miracidia through penetration, or by feeding on the trematode eggs. Asexual reproduction occurs within the mollusc (Ginetsinskaya, Reference Ginetsinskaya1988; Esch et al., Reference Esch, Curtis and Barger2001; Galaktionov & Dobrovolskij, Reference Galaktionov and Dobrovolskij2003; Gordy et al., Reference Gordy, Kish, Tarrabain and Hanington2016). Cercariae are released from molluscs and, once in the environment, they typically seek and infect the second intermediate host, where they encyst to form the metacercaria (Faltynková et al., Reference Faltynkova, Sures and Kostadinova2016).
In the tropical rainforest of Los Tuxtlas, Mexico, a large diversity of trematode species has been reported from different vertebrate groups. At least 57 species of trematodes have been reported from fish, amphibians, reptiles, birds and mammals, and ten of them represented new species (see supplementary table S1). However, with the exception of the recent studies by Velázquez-Urrieta & Pérez-Ponce de León (Reference Velázquez-Urrieta and Pérez-Ponce de León2020, Reference Velázquez-Urrieta and Pérez-Ponce De León2021), no information regarding the life cycle and the morphological characterization of the larval forms of these trematodes is available thus far. The classification of distinct cercarial morphotypes is useful to allocate trematodes into a family, even though in some cases more than one trematode family possess the same cercarial morphotype. For instance, furcocercous cercariae are found in species of Diplostomidae Poirier, 1886, Strigeidae Railliet, 1919 and Clinostomidae Lühe, 1901 (Ginetsinskaja, Reference Ginetsinskaya1988). Nevertheless, the study of larval forms through genetic data is a valuable source of information to understand the species diversity of trematodes by linking larval forms with adults sampled from their vertebrate definitive hosts (e.g. Locke et al., Reference Locke, McLaughlin, Lapierre, Johnson and Marcogliese2011, 2021); even without a formal recognition of the link, cercarial morphotypes might be diagnosed as independent genetic lineages. This is particularly useful for life-history studies partly resolving an ethical concern and avoiding sampling hosts which might be considered as endangered by national and international agencies. Alternatively, by studying the diversity of larval forms of trematodes in their intermediate hosts, it is possible to provide a better estimation of the trematode species diversity (e.g. Gordy et al., Reference Gordy, Kish, Tarrabain and Hanington2016; Gordy & Hanington, Reference Gordy and Hanington2018; Selbach et al., Reference Selbach, Soldanova, Feld, Kostadinova and Sures2020); some species might be found in these hosts while the adult forms have not been reported from their natural definitive hosts in the area. The study of terrestrial, aquatic and semi-aquatic molluscs in a region could be a suitable indicator of trematode species diversity based on the premise that higher mollusc diversity leads to higher trematode diversity, although some exceptions are described in the literature (see Blasco-Costa et al., Reference Blasco-Costa, Faltýnková, Georgieva, Skírnisson, Scholz and Kostadinova2014).
Furthermore, the advancement of molecular tools has facilitated the increase of the DNA reference libraries of several parasite groups, allowing the establishment of the link between larval forms and adults, through the sequencing of some molecular markers from different stages of the life cycle – for example, Locke et al. (Reference Locke, McLaughlin, Lapierre, Johnson and Marcogliese2011) and Blasco-Costa et al. (Reference Blasco-Costa, Poulin and Presswell2015). In particular, the nuclear 28S ribosomal RNA (rRNA) gene accounts for the largest representation of sequence data of species of trematodes (Pérez-Ponce de León & Hernández-Mena, Reference Pérez-Ponce de León and Hernández-Mena2019). However, there is a paucity of the information on the genetic diversity of cercariae released by molluscs, and the distribution patterns of trematodes in their intermediate hosts in different freshwater systems (Faltynkova et al., Reference Faltynkova, Sures and Kostadinova2016). In Mexico, very few studies have characterized the morphology of cercariae in their first intermediate hosts (e.g. Lamothe-Argumedo et al., Reference Lamothe-Argumedo, Meker and Meave-Gallegos1983; Rangel-Ruíz & Lamothe-Argumedo, Reference Rangel-Ruíz and Lamothe-Argumedo1986; Ditrich et al., Reference Ditrich, Scholz, Aguirre-Macedo and Vargas-Vázquez1997; Scholz et al., Reference Scholz, Ditrich and Vargas-Vazquez1996; Barragán-Sáenz et al., Reference Barragán-Sáenz, Sánchez-Nava, Hernández-Gallegos and Salgado-Maldonado2009). To the best of our knowledge, this is the first study addressing the overall genetic diversity and morphological characterization of trematode cercariae in a particular region of Mexico. The main objectives of this work were twofold: to characterize morphologically and molecularly the cercariae of trematodes released from molluscs in two freshwater systems in the tropical rainforest of Los Tuxtlas; and to determine the phylogenetic position of the cercariae through 28S ribosomal DNA (rDNA) sequences with the aim of achieving their taxonomic identification.
Materials and methods
Specimen collection
Ten species of gastropods and one of bivalve were collected in Laguna Escondida (LE) (18°36′00″N, 95°05′26″W), and Laguna Zacatal (LZ) (18°34′55″N, 95°05′ 20″W), Los Tuxtlas, Veracruz, Mexico, during August–September 2018, and January–February 2019 (table 1). Snails and clams were collected by hand on the aquatic vegetation near the shore or using dip nets or a strainer for the sediment at a depth of about 2 m. Specimens were taken alive to the laboratory, placed individually in glass containers with 100 ml of filtered water from the study site, for a period of 16 h of light and 8 h of darkness to stimulate the emergence of cercariae; containers were examined under the stereomicroscope every 6 h for the presence of cercariae; hosts positive to the infection were dissected to search for intra-mollusc trematode stages; snails negative to the infection by cercariae after 36 h were released at the capture site. For morphological study, cercariae were fixed in hot (near boiling) 4% formalin (for the scanning electron microscopy (SEM) study) or in hot (near boiling) tap water; all specimens were preserved in 70% ethanol. For molecular analyses, cercariae were preserved in 100% ethanol. Snails and clams were identified using specific taxonomic keys (Burch, Reference Burch1989; Thorp & Covich, Reference Thorp and Covich1991). The cercariae were identified using a combination of morphological and molecular data.
Table 1. Molluscs collected and infected with trematode cercariae in Los Tuxtlas tropical rainforest.

Morphological study
A photomicrograph of live specimens representing each cercarial morphotype was taken with a Leica DM500 light microscope (Leica Microsystems, Wetzlar, Germany). After fixation, cercariae were stained with neutral red, cleared with 12% glycerol and mounted in Canada balsam between coverslips. Measurements were obtained for some cercarial traits using a drawing tube attached to the microscope; measurements are presented in micrometres. Vouchers of cercarial morphotypes were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City. Cercariae fixed in 4% formalin were used for SEM. They were dehydrated through an ethanol series, critical-point dried, sputter-coated with gold with Q150R Modular Coating System (Ashford, England), and examined at 15 kV with a Hitachi SU1015 SEM (Hitachi, Tokyo, Japan) and S-2460N SEM (Hitachi, Tokyo, Japan). The criterion for classifying the cercarial morphotypes was based on the recognition of traits such as the tail shape, presence/absence of stylet, eye-spots and virgulate organ, as well as the number of suckers (Ginetsinskaja, Reference Ginetsinskaya1988).
Molecular study
For molecular analyses, total DNA was extracted from individual cercariae following the DNAzol protocol (Molecular Research Center, Cincinnati, OH, USA) (Chomczynski et al., Reference Chomczynski, Mackey, Drews and Wilfinger1997). The combination of primers used for amplification and sequencing for the 28S rDNA gene were: 28SL 5′-AAC AGT GCG TGA AAC CGC CTC-3′ (Palumbi, Reference Palumbi, Hillis, Moritz and Mable1996) and URP28S 5′-GCT ATC CTG AGR GAA ACT TCG-3′ (Tkach et al., Reference Tkach, Pawlowski and Mariaux2000); 28Sy 5′-CTA ACC AGG ATT CCC TCA GTA ACG GCG AGT-3′ and 28Sz 5′-AGA CTA CTT TGG TCC GTG TTT CAA GAC-3′ (Hillis & Dixon, Reference Hillis and Dixon1991); and 28Sy 5′-CTA ACC AGG ATT CCC TCA GTA ACG GCG AGT-3′ and 28SL 5′-AAC AGT GCG TGA AAC CGC CTC-3′ (see supplementary table S2); thermal cycling conditions were 1 min at 94°C, then 35 cycles of 45 s at 92°C, 30 s at 50°C and 1.5 min at 72°C, and a final extension of 10 min at 72°C. Polymerase chain reaction products were sequenced using an ABI 3730xl Genetic Analyzer (Thermo Fisher scientific,Waltham, Massachusetts, USA). Sequences were edited and assembled using the software Geneious 5.1.7 (Biomatters Ltd., Auckland, New Zealand). All newly generated sequences were submitted to GenBank.
Phylogenetic analyses
DNA sequences were aligned using MAFFT (Katoh et al., Reference Katoh, Kuma, Toh and Miyata2005; Katoh & Standley, Reference Katoh and Standley2013), using default parameters; alignments were trimmed to the shortest sequence length. Phylogenetic analyses of the 28S rDNA were performed using representative members of nine digenean families: Cryptogonimidae Ward, 1917; Diplostomidae Poirier, 1886; Echinochasmidae Odhner, 1910; Echinostomatidae Looss, 1899; Gorgoderidae Looss, 1899; Heterophyidae Leiper, 1909; Lecithodendriidae Lühe, 1901; Pleurogenidae Looss, 1899; and Strigeidae Railliet, 1919. Analyses were conducted at family level after an initial overall phylogenetic analysis was performed using the alignment of Pérez-Ponce de León & Hernández-Mena (Reference Pérez-Ponce de León and Hernández-Mena2019). Family-level relationships were assessed to better depict the relationships of the newly generated sequence data. Species chosen as outgroups for rooting the trees were selected using the analysis of Pérez-Ponce de León & Hernández-Mena (Reference Pérez-Ponce de León and Hernández-Mena2019). Two phylogenetic approaches were performed, Bayesian Inference and Maximum likelihood; Bayesian Inference analyses were conducted using Markov Chain Monte Carlo in Mr Bayes V 3.1.2 (Ronquist et al., Reference Ronquist, Teslenko and Van Der Mark2012), with the model of evolution GTR+1+ Γ, obtained in jModeltest 0.1.1 (Posada, Reference Posada2008). The chains were run for 1,500,000 generations, sampling trees every 1000 generations. The first 25% of trees were discarded as burn-in and the resulting trees were used to obtain a 50% majority-rule consensus tree. Tracer v1.7.1 (Rambaut et al., Reference Rambaut, Drummond, Xie, Baele and Suchard2018) was used to assess convergence of independent runs. Nodal support was estimated as posterior probabilities. Maximum likelihood analyses were performed in RaxML 6.0 (Stamatakis, Reference Stamatakis2006) with the appropriate evolution model (GTR+1+ Γ) obtained in jModeltest 0.1.1 (Posada, Reference Posada2008). Genetic distances were estimated through uncorrected p-distances using MEGA-X (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018).
The procedure to achieve the identification of cercariae followed that used previously by several authors (Kudlai et al., Reference Kudlai, Stunaenas and Tkach2015; O'Dwyer et al., Reference O'Dwyer, Faltýnková, Georgieva and Kostadinova2015; Donald & Spencer, Reference Donald and Spencer2016; Chontananarth et al., Reference Chontananarth, Tejangkura, Wetchasart and Chimburut2017; Gordy & Hanington, Reference Gordy and Hanington2018; Huston et al., Reference Huston, Cutmore and Cribb2018; Cribb et al., Reference Cribb, Chapman, Cutmore and Huston2019; Onaca et al., Reference Onaca, Graca, Fabrin, Takemoto and Oliveira2020). DNA sequences were generated for individuals representing cercarial morphotypes (and sometimes their intra-molluscan stages), and first screened through the BLAST tool available in GenBank to check that only parasite DNA was amplified, and to preliminary assign each morphotype to a superfamily or family-level group; in some cases, a link was established and morphotypes were assigned to genus and even to species levels. Then, phylogenetic analyses were run for each superfamily or family-level analyses, and the position and sister-group relationships of each morphotype within the tree was established.
Results
A total of 6434 molluscs belonging to two classes, eight families and 11 species were sampled and analysed for infections with digenean trematodes. We examined 4131 individuals from LE and 2303 from LZ (table 1). Seven species of molluscs were sampled in LE and five in LZ. The most abundant were the planorbid gastropod Biomphalaria helophila in LZ, and the cochliopid gastropod Aroapyrgus alleei in LE. The ampullariid gastropod Pomacea catemacensis was the only one found in both localities, although it was not infected with trematodes. Of the 6434 analysed hosts, only 120 harboured trematode infections, resulting in an overall prevalence of 1.86%. Furthermore, only six of the 11 species harboured trematode infections – namely, Melanoides tuberculata, A. alleei, B. heliophila, Hebetancylus excentricus, Ferrissia sp. and Pisidium sp. (see table 1). The species with the highest trematode species richness was A. alleei, with five species.
Twelve cercarial morphotypes we recovered (Furcocercous cercaria I, II, III; Distome cercaria I, II; Xiphidiocercaria, Virgulate cercaria, Ophthalmocercaria, Pleurolophocercous cercaria, Monostome cercaria and Cysticercous cercaria I, II). However, multiple infections were not found; each individual mollusc was parasitized only by one species of trematode at the same time in our samples. Regarding the locality, eight cercarial morphotypes were found in LE and five in LZ.
Morphological descriptions of cercarial morphotypes
Furcocercous cercaria I
Furcocercous cercaria (figs 1a, 2a and 4a) Description based on ten specimens: body small, elongate, 120.6–123.4 (122.1) long, 40.6–41.4 (41.2) wide at equatorial region; tegument spinose, blunt spines distributed as follows: the first third of body without spines, the second possess a circular band and in the third spines are homogeneous; eye-spots spherical, pre-equatorial. Oral sucker subterminal, oval, with two pair of glandular cells. Pharynx large, muscularized. Oesophagus long, narrow; intestinal caeca short. Five pairs of robust penetration glands in two lateral fields, in posterior half of body. Ventral sucker small, spherical, post-equatorial. Excretory vesicle V-shaped. Tail elongated, longer than body, spined, blunt spines, with distribution homogeneous; bifurcated, with longitudinal muscular fibres along central axis, 275.9–285 (284) long, 34–37.1 (35.1) wide; furca length shorter than tail length. In Ferrisia sp., LE.

Fig. 1. Photomicrographs of the cercarial morphotypes released from molluscs in two lakes of Los Tuxtlas, Veracruz. (a) Furcocercous cercaria I; (b) Furcocercous cercaria II; (c) Furcocercous cercaria III; (d) Distome cercaria I; (e) Distome cercaria II; (f) Xiphidiocercaria; (g) Virgulate cercaria; (h) Ophthalmocercaria; (i) Pleurolophocercous cercaria; (j) Monostome cercaria; (k) Cysticercous cercaria I; (l) Cysticercous cercaria II.
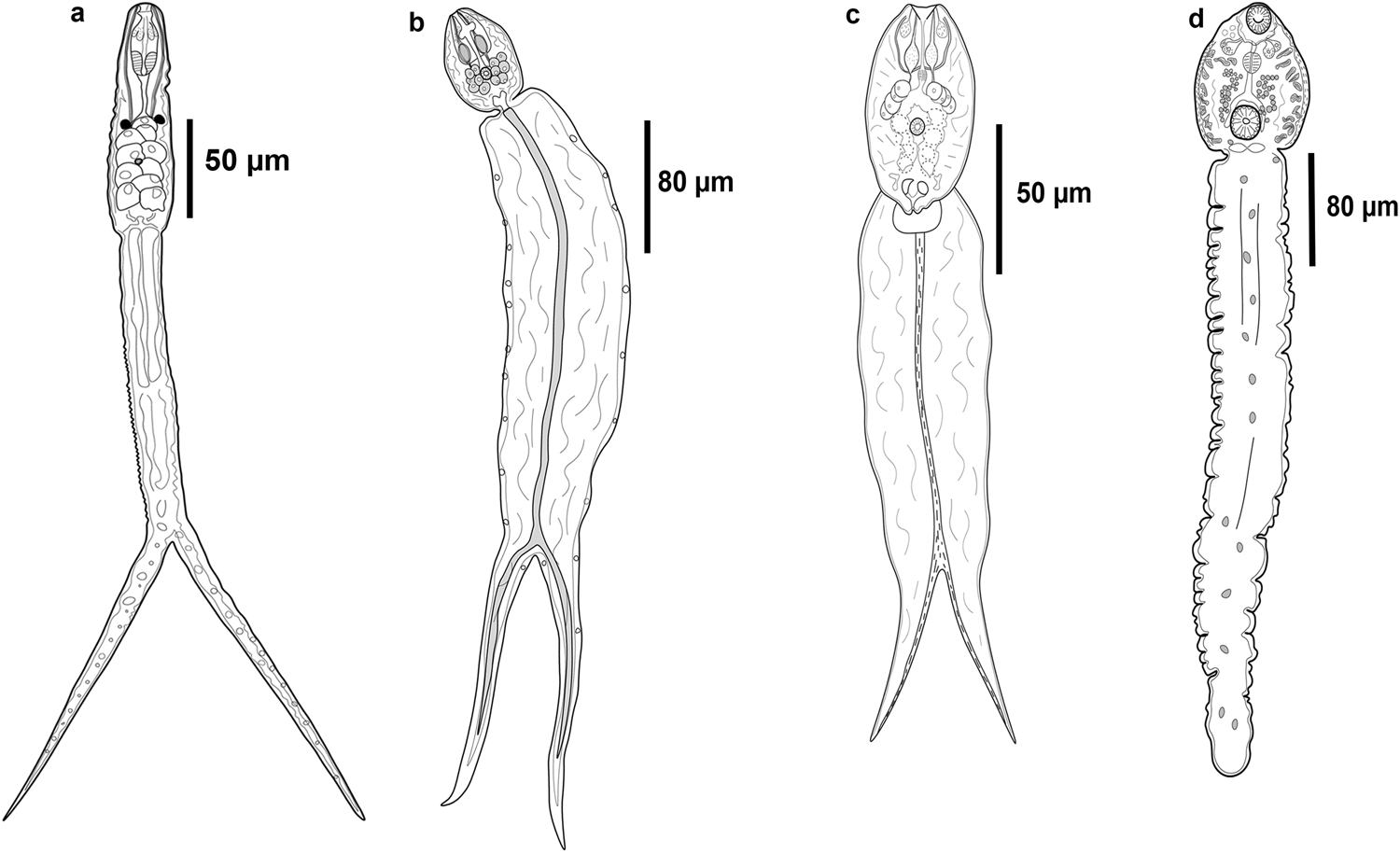
Fig. 2. Line drawings of the cercarial morphotypes released from molluscs in two lakes of Los Tuxtlas, Veracruz. (a) Furcocercous cercaria I; (b) Furcocercous cercaria II; (c) Furcocercous cercaria III; (d) Distome cercaria I.
Furcocercous cercaria II
Furcocercous cercaria II (figs 1b and 2b) Description based on ten specimens: body small, oval, 77.7–79 (78.3) long, 50.3–60.3 (55) wide at equatorial region; tegument spinose, blunt spines homogeneously distributed; eye-spots absent; oral sucker terminal, spherical. Pre-pharynx short and narrow. Pharynx wide. Oesophagus long; intestinal caeca short. Six pairs of spherical penetration glands, distributed in two lateral fields, one on each side of ventral sucker. Ventral sucker small, spherical, post-equatorial, smaller at oral sucker. Excretory vesicle V-shaped. Tail wider and longer than the body, spined, blunt spines, with homogeneous distribution; bifurcated, with longitudinal muscles fibres along the central axis of the tail, 298.2–304 (314) long, 56–65.3 (81) wide; furca length shorter than tail length. In H. excentricus, LE.
Furcocercous cercaria III
Furcocercous cercaria III (figs 1c and 2c) Description based on ten specimens: body small, oval, 76.4–86.3 (81) long, 46.32–50.2 (48.2) wide at equatorial region; tegument spinose, blunt spines with homogeneous distribution; eye-spots absent; oral sucker terminal, spherical, with one pair of glandular cells. Pre-pharynx long and narrow. Pharynx small. Oesophagus short; intestinal caeca long and wide. Four pairs of large penetration glands, distributed in two lateral fields, one on each side of body. Ventral sucker small, spherical, post-equatorial, smaller to oral sucker. Cystogenous glands post-equatorial, overlapping with caeca. Excretory vesicle V-shaped. Tail wider and longer than the body, spined, blunt spines, with homogeneous distribution; bifurcated, with longitudinal muscles fibres along the central axis of the tail, 113.7–228.1 (180.7) long, 73.2–88.3 (80.5) wide; furca length shorter than tail length. In H. excentricus, LE.
Distome cercaria I
Distome cercaria I (figs 1d, 2d and 4b, c) Description based on ten specimens: body spherical, 80.23–96.54 (89) long, 60.56–69.3 (65) wide at equatorial region; tegument smooth. Eye-spots absent. Oral sucker subterminal, spherical, opening directed ventrally. Oral opening with eight nail-shaped spines on anterior border and festoons. Pre-pharynx long; pharynx spherical; oesophagus long. Intestinal caeca short. Two pairs of small penetration glands at pharynx level, distributed in two lateral fields one on each side of body. Ventral sucker spherical, near posterior end, larger than oral sucker; ventral opening surrounded by 37 nail-shaped spines and festoons; three pairs of papillae on internal surface. Cystogenous gland cells distributed in two lateral rows, from pharynx to posterior margin of ventral sucker. Excretory bladder saccular or transversely oval. Tail robust, approximately five times longer than body, 440–501.3 (467.3) long, 80.2–98.3 (89.5) wide. In A. alleei, LE.
Distome cercaria II
Distome cercaria II (figs 1e, 3a and 4d) Description based on ten specimens: body oval, smooth, 97.3–110 (103) long, 50.56–65.3 (59.25) wide at equatorial region; spines and eye-spots absent. Oral sucker subterminal, spherical, opening directed ventrally. Oral opening with ten nail-shaped spines on anterior border and festoons. Pre-pharynx short; pharynx spherical; oesophagus long. Intestinal caeca short. Three pairs of small penetration glands at level of pharynx in two lateral fields on each side of body. Ventral sucker spherical near posterior end, equal size to oral sucker; ventral opening surrounded by 25 nail-shaped spines and festoons. Cystogenous gland cells well-developed, in two lateral rows, between pharynx and ventral sucker. Excretory vesicle saccular. Tail simple, equal size to body length, 80–101 (90.4) long, 20.56–28.3 (24.2) wide. In Biomphalaria helophila, LZ.
Xiphidiocercaria
Xiphidiocercaria (figs 1f and 3b) Description based on ten specimens: body small, elongate, 90.6–91.7 (91.3) long, 44.3–61.8 (55.5) wide at equatorial region; tegument without spines. Eye-spots absent. Oral sucker subterminal, spherical, opening ventrally. Oral stylet well-developed, lanceolate. Pharynx globular. Oesophagus inconspicuous, intestinal caeca short. Three pairs of large penetration glands, equatorial, distributed in two lateral fields on each side of body. Ventral sucker spherical, smaller than oral sucker, post-equatorial. Cystogenous gland cells in the posterior end; excretory vesicle Y-shape. Tail small, simple, 31.3–48.8 (42.8) long, 20.2–27.2 (23.1) wide. In A. alleei, LE.

Fig. 3. Line drawings of the cercarial morphotypes released from molluscs in two lakes of Los Tuxtlas, Veracruz. (a) Distome cercaria II; (b) Xiphidiocercaria; (c) Virgulate cercaria; (d) Ophthalmocercaria; (e) Pleurolophocercous cercaria; (f) Monostome cercaria; (g) Cysticercous cercaria I; (h) Cysticercous cercaria II.
Virgulate cercaria
Virgulate cercaria (figs 1g and 3c) Description based on 20 specimens: body small, elongate, 128.3–204.2 (159.9) long, 62.3–90.5 (76.5) wide at equatorial region; tegument spinose, blunt spines, with homogeneous distribution; small ciliated papillae among spines; eyes-spots absent. Oral sucker subterminal, spherical, oral aperture opening ventrally, one pair of virgulae present. Oral stylet well-developed, lanceolate, needle shaped. Pharynx globular. Oesophagus inconspicuous; intestinal caeca short. Three pairs of penetration glands at level of ventral sucker, in two lateral fields on each side of body. Ventral sucker spherical, smaller than oral sucker, post-equatorial. Excretory vesicle Y-shaped in posterior extremity of body. Tail simple, shorter than body, without spines, 76.4–153.2 (115.9) long, 14–33.1 (21.8) wide. In A. alleei, LE.
Ophthalmocercaria
Ophthalmocercaria (figs 1h, 3d and 4e, i) Description based on ten specimens: body small, elongate, 120.1–138 (127) long, 70.3–75.9 (73.3) wide at equatorial region; tegument spinose, spines blunt, with homogeneous distribution; four pairs of small ciliated papillae on ventral region; eyes-spots large, spherical to oval. Oral sucker subterminal, spherical, oral aperture opening ventrally; nine hooks on dorsal border of oral opening; six papillae on ventral border of oral opening. Pharynx inconspicuous, globular. Oesophagus short, intestinal ceca short. Four pairs of large penetration glands, oval, in two lateral fields on each side of body. Ventral sucker absent. Cystogenous gland cells distributed along body. Excretory vesicle oval-shaped, in posterior extremity of body. Tail simple without spines, equal large than body 161.6–182.2 (170.1) long, 21.8–34.7 (26.8) wide. In M. tuberculata, LZ.

Fig. 4. SEM photomicrographs of the cercarial morphotypes released from molluscs in two lakes of Los Tuxtlas, Veracruz. (a) Body of Furcocercous cercaria I; (b) body of Distome cercaria I; (c) oral sucker of Distome cercaria I; (d) ventral sucker of Distome cercaria II; (e) body of Ophthalmocercaria; (f) body of Pleurolophocercous cercaria; (g) body of Monostome cercaria; (h) body of Cysticercous cercaria I; (i) oral sucker of Ophthalmocercaria; (j) oral sucker of Pleurolophocercous cercaria; (k) oral sucker of Monostome cercaria; (l) oral sucker of Cysticercous cercaria I.
Pleurolophocercous cercaria
Pleurolophocercous cercaria (figs 1i, 3e and 4f, j) Description based on ten specimens: body small, bell-shaped, 140.3–169.6 (155.4) long, 50–100.2 (80.2) wide at equatorial region; tegument spinose in anterior half from anterior end to level of eye-spots, spines blunt; eyes-spots large, oval to spherical. Oral sucker subterminal, spherical. Ten large circumoral spines on dorsal border of oral opening. Pharynx, oesophagus and intestinal caeca not discernible in our specimens. Ventral sucker weakly developed, spherical, equatorial. Six pairs of penetration glands in two lateral fields, one on each side of body. Cystogenous glands distributed along body. Excretory vesicle large, Y-shaped. Tail longer than body, curved dorsoventrally, with a lateral fin-fold, 310–390 (337.3) long, 20.3–38.9 (27.3) wide. In A. alleei, LE.
Monostome cercaria
Monostome cercaria (figs 1j, 3f and 4g, k) Description based on ten specimens: body small, elongate, 98.9–110.5 (103.5) long, 48.23–50.7 (49.5) wide at mid-hindbody; tegument spinose, spines lanceolate, with homogeneous distribution; two large eyes-spots, spherical. Oral sucker subterminal, spherical, oral aperture directed ventrally, with four conspicuous hooks on antero-dorsal border of oral opening in one horizontal row. Pharynx globular, small. Oesophagus and intestinal ceca inconspicuous. Seven pairs of large penetration glands, in two lateral fields on each side of body, forming two clusters. Ventral sucker absent. Excretory vesicle oval. Tail simple, shorter than body, without spines, 80.45–87.2 (83.7) long, 14.5–17.45 (15.7) wide. In A. alleei, B. helophila and M. tuberculata, LE and LZ.
Cystocercous cercaria
Cystocercous cercaria (figs 1k, 3g and 4h, l) Description based on ten specimens: anterior chamber hollow, oval, with an opening in anterior region. Total length 1090.5–1500.2 (1370.3). Apparently without prominent structures. A pyramidal mass of granular cells arising from the base at junction with the tail. Tail longer than the chamber, cylindrical, tapering from anterior end, almost equal to that of the chamber to form a bluntly rounded posterior end; transparent, with nuclei in greater concentration near the anterior end. Cercaria body small, elongate, 136.3–148.4 (145.4) long, 55–57.2 (55.1) wide at equatorial region; tegument lacking spines; eye-spots lacking. Sensorial papillae distributed ventrally along body. Oral sucker subterminal, spherical, oral aperture opening ventrally, with numerous sensorial bristles along exterior border. Dumb-bell-shaped papillae surrounding oral sucker opening. Oral stylet well-developed, lanceolate, rounded posteriorly. Pharynx absent. oesophagus long. Intestinal caeca short reaching posterior border of ventral sucker. Six pairs of penetration glands, pre-acetabular, in two lateral clusters on each side of body. Ventral sucker spherical, larger than oral sucker, post-equatorial. Excretory bladder Y-shaped, cytogenous gland cells arranged in two clusters. Excretory pore at posterior end. In Pisidium sp., LZ.
Cystocercous cercaria II
Cystocercous cercaria II (figs 1l and 3h) Description based on ten specimens: anterior chamber hollow, spherical, without anterior opening. Total length 905.3–960.2 (921.1). Without prominent structures. A rectangular mass of granular cells arises from the base at junction with the tail. Tail cylindrical, tapering posteriorly; bluntly rounded posterior end. Transparent body. The chamber contains cercarial body 115.2–139.2 (127.2) long, 147–136.4 (141.9) wide. Body small, elongate to oval, 65.2–80.4 (73.6) long, 42.3–49.2 (44.8) wide at equatorial region; tegument without spines, with small papillae; eyes-spots lacking. Oral sucker subterminal, spherical, oral aperture directed ventrally, sensorial bristles not observed. Oral stylet well-developed, lanceolate. Pharynx absent. Oesophagus long. Intestinal caeca short. Six pairs of transversally elongated penetration glands, pre-acetabular, in two lateral clusters on each side of body. Ventral sucker spherical, post-equatorial, sensorial bristles not observed, larger than oral sucker. Excretory vesicle Y-shaped. Excretory pore at posterior end. In Pisidium sp., LZ.
Molecular identification of cercarial morphotypes
A preliminary BLAST search of the GenBank nucleotide database showed that the newly generated sequences belong to eight families of digeneans, namely Diplostomidae, Strigeidae, Echinochasmidae, Gorgoderidae, Heterophyidae, Lecithodendriidae, Pleurogenidae and Cryptogonimidae. To further corroborate the allocation of each morphotype into a genus, sequences were included in a comprehensive phylogenetic analysis of the Digenea. Morphotypes recognized morphologically were allocated into ten genera (table 2): Apharyngostrigea Ciurea, 1927, Ascocotyle Looss, 1899, Centrocestus Looss, 1899, Echinochasmus Dietz, 1909, Gorgoderina Looss, 1899, Langeronia Caballero & Bravo, 1949, Lecithodendrium Looss, 1896, Oligogonotylus Watson, 1976, Phyllodistomum Braun, 1899 and Posthodiplostomum Dubois, 1936. To further identify the cercarial morphotypes to the lowest taxonomic level, particular phylogenetic analyses were built for each family group using available 28S rDNA sequences from GenBank. The 12 cercarial morphotypes diagnosed morphologically resulted in the recognition of 16 putative trematode species based on molecular phylogenetic analyses of the 28S rRNA gene, ten as parasites of birds, three of freshwater fish, two of amphibians and one of mammals (table 2).
Table 2. Summary data of cercarial morphotypes in molluscs of the tropical rainforest of Los Tuxtlas, Veracruz, Mexico.
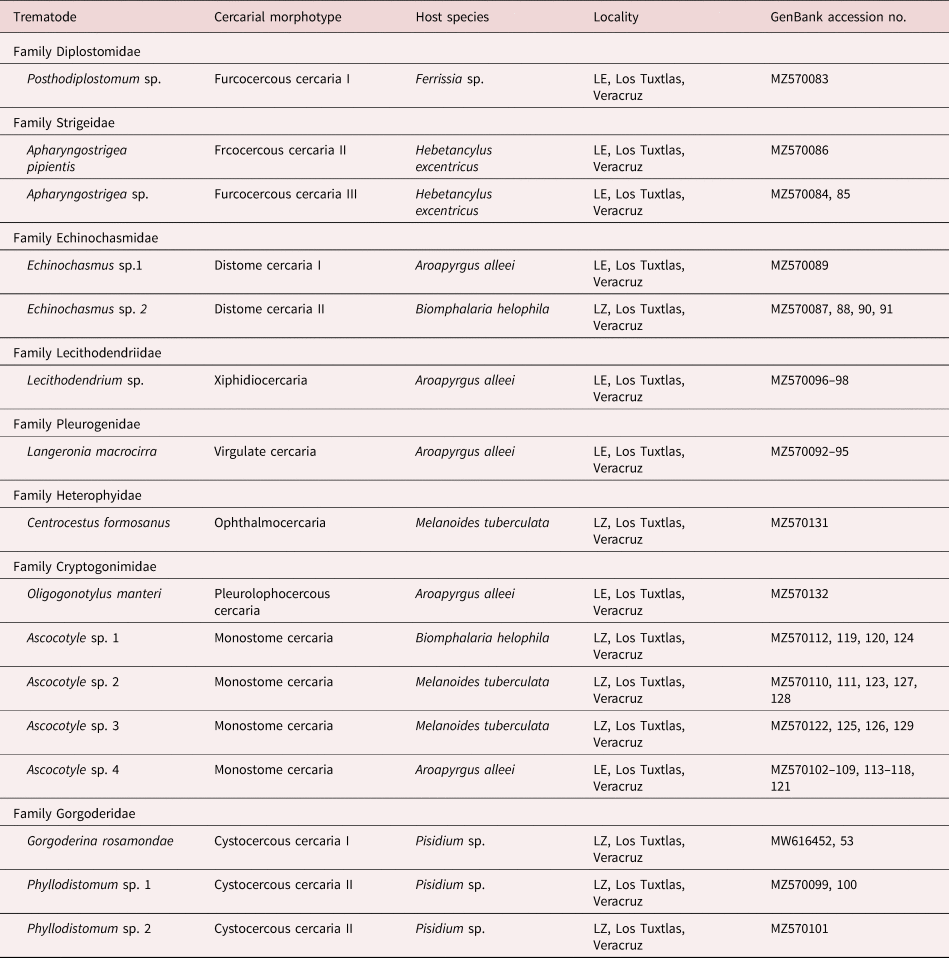
LE, Laguna Escondida; LZ, Laguna Zacatal.
The alignment of the phylogenetic analyses of the families Diplostomidae and Strigeidae) consisted of 1303 bp, including the newly generated sequences and those available in GenBank for some members of the superfamily Diplostomoidea (fig. 5a). Mesostephanus microbursa Caballero, Grocott & Zerecero, 1953 and Braunina cordiformis Wolf, 1903 were selected as outgroups. The sequence of the furcocercous cercariae type I (ex Ferrissia sp., one isolate) was placed as sister of Posthodiplostomum sp. Genetic divergence among congeners was 3.2%. The sequences of the furcocercous cercariae type II (H. excentricus, one isolate) and III (two isolates) appeared as sister taxa of several species of Apharyngostrigea and Parastrigea Szidat, 1928, with a very low genetic divergence among the novel sequences and Apharyngostrigea (0.23%) (fig. 5a).
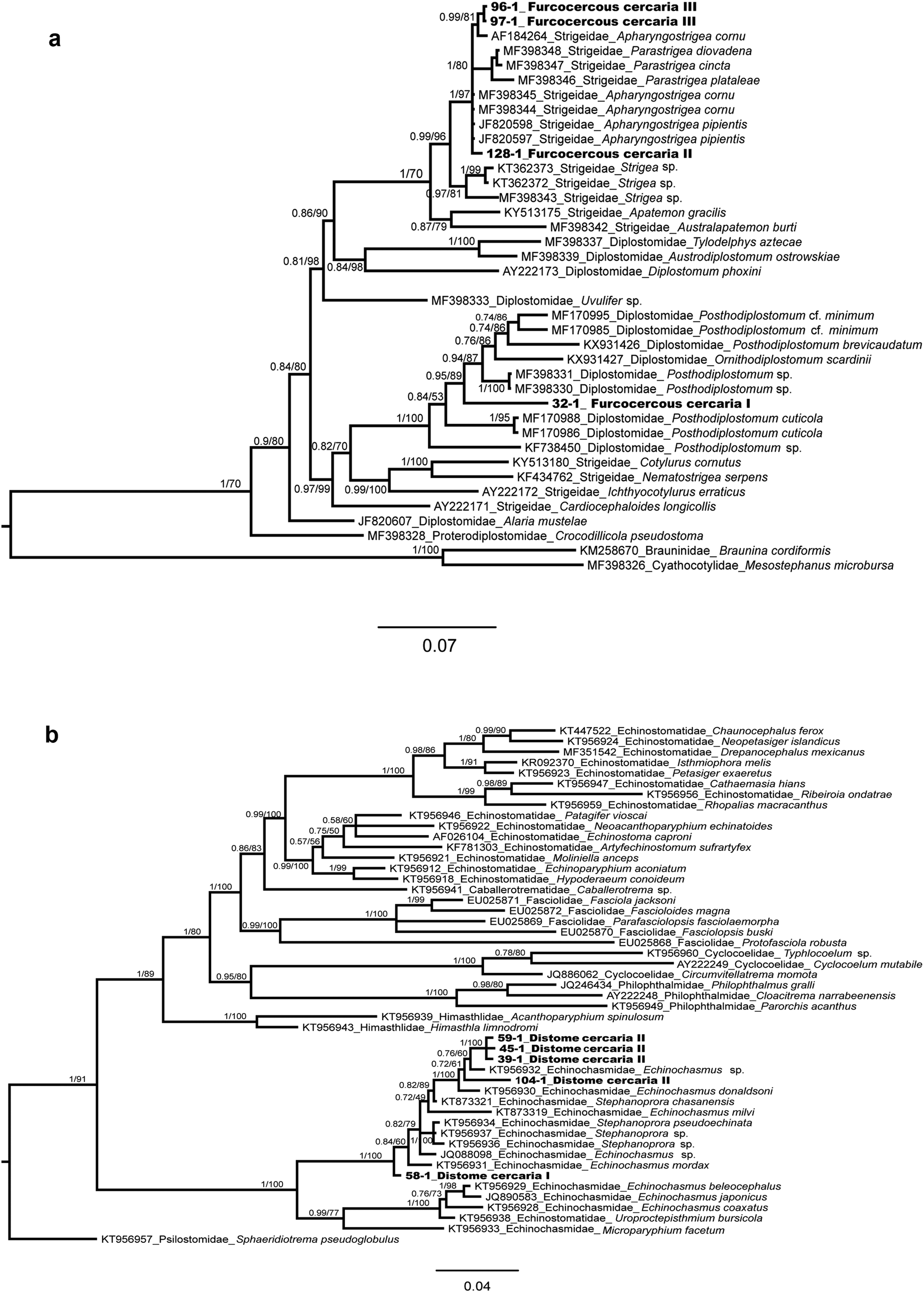
Fig. 5. Bayesian phylogenetic trees of the 28S rRNA showing the phylogenetic positions of Furcocercous cercaria and Distome cercaria morphotypes released by snails from Los Tuxtlas. (a) Partial phylogenetic tree of Diplostomatoidea; (b) partial phylogenetic tree of Echinostomatoidea.
In the phylogenetic analysis of the family Echinochasmidae, the alignment consisted of 1225 bp, including the newly generated sequences and those available in GenBank for some members of the superfamily Echinostomatoidea (fig. 5b); sequences of the Distome cercaria I (ex A. alleei, one isolate) and Distome cercaria II (ex B. helophila, four isolates) appeared as sister taxa of species of the genera Echinochasmus Dietz, 1909 and Stephanoprora Odhner, 1902, showing 0.5–0.6% of genetic divergence.
For the phylogenetic analysis of the families Lecithodendriidae and Pleurogenidae, the alignment consisted of 1290 bp, including the newly generated sequences and those available in GenBank for some members of superfamily Microphalloidea (fig. 6a); sequences of Xiphidiocercaria (ex A. alleei, three isolates) appeared as the sister species of Lecithodendrium spp., with a genetic divergence of 2.2%. Instead, sequences of the virgulate cercaria (ex A. alleei, four isolates) were nested in a clade with Langeronia macrocirra (=Loxogenes macrocirra) Caballero & Bravo, 1949, with null genetic divergence and showing conspecificity (fig. 6a).

Fig. 6. Bayesian phylogenetic trees of the 28S rRNA showing the phylogenetic position of Virgulate cercaria, Xiphidiocercaria and Cystocercous cercaria morphotypes released by snails or clams from Los Tuxtlas. (a) Partial phylogenetic tree of superfamily Microphalloidea; (b) partial phylogenetic tree of the superfamily Gorgoderoidea.
The phylogenetic analysis of the family Gorgoderidae included an alignment of 1293 bp, considering the newly generated sequences and those available in GenBank for some members of the superfamily Gorgoderoidea (fig. 6b). Sequences of the Cystocercous cercaria I were identical to Gorgoderina rosamondae Velázquez-Urrieta & Pérez-Ponce de León, 2020, whereas the single sequence of the Cystocercous cercaria type II (ex Pisidium sp., three isolates) corresponded genetically with the genus Phyllodistomum; however, one isolate showed a very low genetic divergence with Phyllodistomum inecoli Razo-Mendivil, Pérez-Ponce de León & Rubio-Godoy, 2013 (0.2%) and was considered conspecific (fig. 6b). The other two isolates of that cercarial morphotype represented a separate species.
Finally, the phylogenetic analysis cercarial morphotypes included in the families Cryptogonimidae and Heterophyidae consisted of 1298 bp, including the newly generated sequences and those available in GenBank for some members of superfamily Opisthorchioidea (fig. 7); sequences of the Pleurolophocercous cercaria (ex A. alleei, one isolate) corresponded genetically with Oligogonotylus manteri Watson, 1976; sequences of the Ophthalmocercaria (ex M. tuberculata two isolates) were identical to Centrocestus formosanus Nishigori, 1924. Twenty-eight sequences of the Monostome cercaria were obtained, including four isolates from B. heliophila, five isolates from M. tuberculata and 19 isolates from A. alleei. All these sequences were retrieved as four independent monophyletic clades and appeared as sister taxa of different species of Ascocotyle Looss, 1899, although conspecificity with some species was not evidenced for either of them because genetic divergence values varied were very high and varied between 5 and 7%. Overall, molecular phylogenetic analyses of the cercarial morphotypes yielded the identification of eight to genus level and four to species level (G. rosamondae, L. macrocirra, O. manteri, P. inecoli).

Fig. 7. Bayesian phylogenetic tree of the 28S rRNA of the superfamily Opisthorchioidea (in part), showing the phylogenetic position of Monostome cercaria, Ophthalmocercaria and Pleurolophocercus cercaria released by snails from Los Tuxtlas.
Discussion
The present study represents a fine-scale morphological and molecular analysis of the trematode diversity in two freshwater habitats of Los Tuxtlas tropical rainforest. Overall, of the 11 species of molluscs studied, six harboured trematode infections; however, our results showed that each locality possesses their own mollusc and digenean fauna. Traditionally, the recognition of cercarial diversity rely on detailed morphological descriptions, sometimes accompanied with the description of intra-molluscan stages (e.g. Scholz et al., Reference Scholz, Aguirre-Macedo and Salgado-Maldonado2001; Gilardoni et al., Reference Gilardoni, Etchegoin, Diaz, Ituarte and Cremonte2011; Veeravechsukij et al., Reference Veeravechsukij, Namchote, Neiber, Glaubrecht and Krailas2018). The modern approach to describe larval trematode diversity has been successfully used by several authors (see Kudlai et al., Reference Kudlai, Stunaenas and Tkach2015; O'Dwyer et al., Reference O'Dwyer, Faltýnková, Georgieva and Kostadinova2015; Donald & Spencer, Reference Donald and Spencer2016; Chontananarth et al., Reference Chontananarth, Tejangkura, Wetchasart and Chimburut2017; Gordy & Hanington, Reference Gordy and Hanington2018; Huston et al., Reference Huston, Cutmore and Cribb2018; Cribb et al., Reference Cribb, Chapman, Cutmore and Huston2019; Onaca et al., Reference Onaca, Graca, Fabrin, Takemoto and Oliveira2020). We followed such approach and were able to allocate the 12 cercarial morphotypes into 16 putative species, using a combination of morphological and molecular data along with other sources of information such as the previous records of trematodes in the surrounding area, host association and geographical distribution.
Families Diplostomidae and Strigeidae
In the phylogenetic tree (fig. 5a), three morphotypes were resolved as members of the families Diplostomidae and Strigeidae – that is, the Furcocercous cercariae types I, II and III. The first one was nested within a group of species of the genera Posthodiplostomum Dubois, 1936 and Ornithodiplostomum Dubois, 1936, with high nodal support value. In Veracruz, at least eight species of freshwater fish have been reported as hosts of the metacercariae of Posthodiplostomum minimum (see Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Prieto and Mendoza-Garfias2007); adults of P. minimum were reported from ardeids of Lake Catemaco, a locality 31 km south of LE (Lamothe-Argumedo & Pérez Ponce de León, Reference Lamothe-Argumedo and Pérez-Ponce de León1986). Sequences of the 28S rRNA gene of those specimens are not available yet and are required to confirm they are conspecific with our samples. However, due to the phylogenetic position on the tree, we concluded that our specimens belong in the genus Posthodiplostomum. We cannot ascertain if the specimens from Los Tuxtlas belong to the species P. minimum. An ongoing study on the genetic diversity of larval Posthodiplostomum in Middle America shows higher richness of species in the genus as previously considered.
The second and third morphotypes (Furcocercous cercariae type II and III) were resolved within the family Strigeidae sensu lato, in a group of species of the genera Apharyngostrigea Ciurea, 1927, and Parastrigea; these relationships were also supported with a high nodal support value. In Veracruz, two species have been reported and sequenced from ardeid fish-eating birds (Hernández-Mena et al., Reference Hernández-Mena, García-Prieto and García-Varela2014, Reference Hernández-Mena, García-Varela and Pérez-Ponce de León2017), Apharyngostrigea cornu (Zeder, 1800) and Parastrigea diovadena Dubois & Macko, 1972. Particularly in the region of Los Tuxtlas, adult forms of Apharyngostrigea sp. from Egretta thula were recorded by Lamothe-Argumedo et al. (Reference Lamothe-Argumedo, Pérez-Ponce de León, García-Prieto, González, Dirzo and Vogt1993). Figure 5a depicts the interrelationships among A. cornu, A. pipientis (Faust, 1918) and P. diovadena in an unresolved polytomy, although the genetic distance between our samples and A. cornu is the lowest (0.23%). We identified our specimens as members of Apharyngostrigea. A recent study (Locke et al., Reference Locke, Drago, López-Hernández, Chibwana, Núñez, Dam, Achinelly, Johnson, Alves de Assis, Lane de Melo and Pinto2021) revealed that previous sequences identified as A. cornu from Mexico match those of A. pipientis, a species widely distributed across the world. Most likely, the furcocercous cercariae released from H. excentricus characterized in our study correspond with A. pipientis; still, DNA sequences of adult forms from fish-eating birds are required to confirm the hypothesis.
Family Echinochasmidae
In the phylogenetic tree (fig. 5b), two cercarial morphotypes were resolved as members of the family Echinochasmidae: Distome cercaria I and II. Both morphotypes were nested within a group of species of the genera Echinochasmus and Stephanoprora, which were not yielded as monophyletic, with high nodal support value. The Distome cercaria I was nested within species of Echinoschasmus spp. The metacercariae of Echinochasmus leopoldinae Scholz, Ditrich & Vargas, 1996 has been reported infecting several freshwater fish species from Los Tuxtlas tropical rainforest and neighbouring areas (see Scholz et al., Reference Scholz, Ditrich and Vargas-Vazquez1996; Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Prieto and Mendoza-Garfias2007). The species was sampled from Balzapote, approximately 6.3 km from LE; however, sequences of the 28S rRNA gene of that species are not currently available for comparison. Whether or not they correspond to E. leopoldinae considering the previous reports based on morphology needs to be determined when sequences of the 28S rRNA gene and other molecular markers become available. Sequences of the Distome cercaria II occupy a different position within the phylogenetic tree, but still belong in Echinochasmus; these sequences show a small genetic distance (0.5–0.6%) with respect to Echinochasmus sp. and E. donaldsoni; the latter species was described from Podilymbus podiceps L. in the USA (Tkach et al., Reference Tkach, Kudlai and Kostadinova2015). We cannot confirm that our sequences belong in one of these taxa. Interestingly, the two putative species of Echinochasmus were sampled from different snail species and different localities.
Families Lecithodendriidae and Pleurogenidae
In the phylogenetic tree (fig. 6a), two cercarial morphotypes were resolved as belonging to the families Lecithodendriidae and Pleurogenidae: the Xiphidiocercaria and the Virgulate cercaria. The fist morphotype appeared as the sister species of Lecithodendrium spp., and these relationships were supported with a high nodal support values. Species of Lecithodendridae are parasites of insectivorous vertebrates and they use prosobranch molluscs as first intermediate hosts. Cercariae released from the snails encyst as metacercariae in aquatic insect larvae; these insects and then ingested by an insectivorous host and the life cycle is completed (Enabulele et al., Reference Enabulele, Lawton, Walker and Kirk2018). Species of Lecithodendrium are parasites of insectivorous bats, although no species has been reported in Mexico thus far (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Prieto and Mendoza-Garfias2007). Ditrich et al. (Reference Ditrich, Scholz, Aguirre-Macedo and Vargas-Vázquez1997) reported Xiphidiocercaria sp. 3 as probably belonging to Lecithodendridae, with no further discussion. Reports of at least three species of the genus Paralecithodendrium Travassos, 1921 parasitic in bats are available in localities of central Mexico. Since our sequences nest in a monophyletic clade highly supported along with other species of Lecithodendrium, we inferred that our specimens belong in that genus, although we cannot confirm the identification at species level based on the genetic distance with respect to these species. The Xiphidiocercaria sp. 3 reported by Ditrich et al. (Reference Ditrich, Scholz, Aguirre-Macedo and Vargas-Vázquez1997) as Lecithodendriidae? is morphologically different from the one reported in our study. Our specimens possess three pairs of large penetration glands, and the ventral sucker is located posteriorly to these glands; instead, Xiphidiocercaria sp. 3 possesses four pairs of penetration glands, and the ventral sucker is located anteriorly to penetration glands. Furthermore, the topology of the phylogenetic analysis demonstrate also that they do not belong in Paralecithodendrium, which is resolved as the sister group of Lecithodendrium, confirming the results obtained by Enabulele et al. (Reference Enabulele, Lawton, Walker and Kirk2018).
The second morphotype, the Virgulate cercaria, is resolved within the Pleurogenidae as the sister taxa of L. macrocirra (=L. macrocirra), with high nodal support. The report of this cercarial morphotype was recently published by Velázquez-Urrieta & Pérez Ponce de León (Reference Velázquez-Urrieta and Pérez-Ponce de León2020), who inferred the life cycle of L. macrocirra in Los Tuxtlas tropical rainforest. The molecular and morphological information was used to link the larval forms with the adult stage of the trematode, and the transmission pathway of the species was elucidated. Adults of L. macrocirra have been reported in frogs in LE (Paredes-Calderón et al., Reference Paredes-Calderón, León-Règagnon and García-Prieto2004; Paredes-León et al., Reference Paredes-León, García-Prieto, Guzmán-Cornejo, León-Règagnon and Pérez2008; Martínez-Salazar & León-Règagnon, Reference Martínez-Salazar and León-Règagnon2010).
Family Gorgoderidae
Two morphotypes were resolved as members of the family Gorgoderidae (fig. 6b): the Cystocercous cercaria type I and II. The Cystocercous cercaria I is the larval form of G. rosamondae Velázquez-Urrieta & Pérez-Ponce de León, 2021. This life cycle of the species was recently inferred by Velázquez-Urrieta & Pérez-Ponce de León (Reference Velázquez-Urrieta and Pérez-Ponce De León2021). In the region of Los Tuxtlas, three additional species of Gorgoderina have been reported – namely, Gorgoderina festoni Mata-López & León Règagnon, 2005, Gorgoderina parvicava (Travassos, 1922) and Gorgoderina sp. (reported as Gorgoderina attenuata (Stafford, 1902) (Guillén-Hernández et al., Reference Guillén-Hernández, Salgado-Maldonado and Lamothe-Argumedo2000; Paredes-Calderón et al., Reference Paredes-Calderón, León-Règagnon and García-Prieto2004; Mata-López & León-Règagnon, Reference Mata-López and León-Règagnon2005). The second morphotype, Cystocercous cercaria type II, was nested within a group of species of Phyllodistomum, with high nodal support value; it appears as sister taxon of P. inecoli. This species has been reported as adult in freshwater fishes, particularly poeciliids in Veracruz, but also in other states across south-eastern Mexico (Razo-Mendivil et al., Reference Razo-Mendivil, Pérez-Ponce de León and Rubio-Godoy2013); the 28S rDNA sequences are very similar to P. inecoli, exhibiting low genetic distance (0.2%). This lead us to conclude that our specimens represent the cercariae of P. inecoli.
Families Cryptogonimidae and Heterophyidae
In the phylogenetic tree (fig. 7), the Pleurolophocercous cercaria morphotype was resolved as a member of the family Cryptogonimidae and corresponded genetically with O. manteri because sequences were identical to those downloaded from GenBank. This species of trematode is commonly found in Middle American freshwater fishes of the family Cichlidae (Watson, Reference Watson1976; Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Prieto and Mendoza-Garfias2007; Razo-Mendivil et al., Reference Razo-Mendivil, Rosas-Valdez and Pérez Ponce de León2008), which serve both as the second intermediate and definitive hosts (Scholz et al., Reference Scholz, Lavadores, Vargas, Mendoza, Rodriguez and Vivas1994). Morphologically, the cercariae of O. manteri is very similar to that described by Scholz et al. (Reference Scholz, Lavadores, Vargas, Mendoza, Rodriguez and Vivas1994), except by the fact that these authors reported the presence of seven pairs of penetration glands, and we only observed six in our specimens. Another difference is the first intermediate host. Scholz and co-workers elucidated the life cycle of the species in the Yucatan peninsula, and Pyrgophorus coronatus Pfeiffer was recognized as the intermediate host; in our study, we report the species in the gastropod A. alleei.
Two additional morphotypes were uncovered in our phylogenetic analysis (fig. 7), the Ophtalmocercaria and a Monostome cercaria; both yielded as members of the family Heterophyidae. The Ophthalmocercaria was genetically identical to C. formosanus. The metacercariae of C. formosanus has been reported in more than 60 freshwater fish species across Mexico, and represents an invasive species introduced to Mexico along with its first intermediate host, the thiarid snail M. tuberculata (Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Prieto and Mendoza-Garfias2007; Aguilar-Aguilar et al., Reference Aguilar-Aguilar, Martínez-Aquino, Pérez-Rodríguez and Pérez-Ponce-de-León2009). In the area of Los Tuxtlas, the metacercariae of C. formosanus has been reported in five species of freshwater fishes of the families Mugilidae, Poeciliidae and Cichlidae (Salgado-Maldonado et al., Reference Salgado-Maldonado, Aguilar-Aguilar, Cabanãs-Carranza, Soto-Galera and Mendoza-Palmero2005).
The third morphotype, the Monostome cercaria, was nested within a group of species of the genus Ascocotyle, with high nodal support values. As adults, species of Ascocotyle are parasites of fish-eating birds. Even though we observed no discernible morphological differences among specimens of this cercarial morphotype, they formed four separate clades, probably corresponding to four species; one appears as the sister species of Ascocotyle patagoniensis Hernández, Montero, Crespo, Garcia, Raga & Aznar, 2012, albeit with a large genetic distance (5.2–6.2%); our specimens are clearly a separate species. These two are the sister group of a clade formed by five individuals that seem to represent a separate species, diverging from the other two by 6.9–7%. Another clade is formed by two subclades, one formed by four sequences, representing a separate species, sister to Ascocotyle pindoramensis Scholz, Santos & Portes, 2006, and 15 additional sequences of the Monostome cercaria, which also represent a separate species. The genus Ascocotyle is particularly species-rich in México. Twelve species allocated into three subgenera (i.e. Ascocotyle, Leighia and Phagicola) have been reported México, all as metacercariae, and seven also as adults (see Pérez-Ponce de León et al., Reference Pérez-Ponce de León, García-Prieto and Mendoza-Garfias2007). Unfortunately, no sequences of any of these species have been generated in previous studies. Three species – namely, Ascocotyle (Ascocotyle) tenuicollis Price, 1935, Ascocotyle (Phagicola) diminuta Stunkard & Haviland, 1924 and Ascocotyle (Phagicola) nana Ransom, 1920 – are widely distributed in freshwater fishes and fish-eating birds across south-eastern Mexico. We cannot ascertain whether the sequences generated in our study correspond to any of the species previously reported until DNA sequences of adults are generated and the molecular link between the cercariae and the adults is established. In Veracruz alone, where the tropical rainforest of Los Tuxtlas lies, six species of Ascocotyle have been reported (see supplementary table S1).
Trematode species diversity in Los Tuxtlas
Several parasite surveys have been conducted in Los Tuxtlas tropical rainforest; at least 57 trematode species have been reported in 35 species of vertebrates (supplementary table S1); this might be considered a large species richness because the number of analysed host species only represents 3.3% of the vertebrate fauna of the region. In total, 877 species of vertebrates have been reported, including 80 freshwater fishes, 44 amphibians, 118 reptiles, 507 birds and 128 mammals (González et al., Reference González, Dirzo and Vogt1997; López, Reference López, Reynoso, Coates and Vázquez2017). Of the 57 species of trematodes, only two cercariae have been morphologically and genetically characterized (Velázquez-Urrieta & Pérez-Ponce de León, Reference Velázquez-Urrieta and Pérez-Ponce de León2020, Reference Velázquez-Urrieta and Pérez-Ponce De León2021). Our study reported 16 species of trematodes, of which only four corresponded with adult forms previously reported in vertebrates of the area – namely, L. macrocirra, G. rosamondae, both in amphibians, C. formosanus and O. manteri in freshwater fishes. The remaining 12 putative species were allocated up to genus level, and their identity requires further molecular work; still, adults of the 12 species must be in the study region since their cercariae are infecting molluscs in the two freshwater habitats. Our study increased the trematode species diversity of Los Tuxtlas to 73 species; of these, 23 are parasites of freshwater fish, 19 of amphibians, 16 of birds, five of reptiles and ten of mammals. However, our expectation was to find a larger trematode species diversity in the studied molluscs, including not just a higher prevalence of infection and species richness, but also showing correspondence with the adults that have been found in vertebrates of the tropical rainforest. We speculate that there must be a seasonality in the distribution of cercariae among individual molluscs because we noticed a different composition of cercariae in the two sampling seasons. However, more freshwater habitats need to be sampled within the tropical rainforest to fully assess the diversity of molluscs that would serve as first intermediate hosts of trematodes. Clearly, the inventory of the trematode fauna in the region is far from complete. Still, many species of vertebrates remain unstudied.
Most of the species uncovered in this study exhibit a classic three-host life cycle, with the exception of O. manteri, which has been shown to have the capacity to abbreviate its life cycle since fish may serve as second intermediate host and definitive host (Scholz et al., Reference Scholz, Lavadores, Vargas, Mendoza, Rodriguez and Vivas1994). Most of the species we report in this study use a wide variety of invertebrates and vertebrates as second intermediate host. For instance, species in the genera Apharyngostrigea, Ascocotyle, Centrocestus, Echinochasmus and Oligogonotylus encyst in fish; Langeronia, Phyllodistomum and Lecithodendrium encyst in aquatic insect larvae; and Gorgoderina encyst in tadpoles and odonates (Scholz et al., Reference Scholz, Lavadores, Vargas, Mendoza, Rodriguez and Vivas1994, Reference Scholz, Ditrich and Vargas-Vazquez1996; Scholz & Salgado-Maldonado, Reference Scholz and Salgado-Maldonado2000; Bolek et al., Reference Bolek, Snyder and Janovy2009; Locke et al., Reference Locke, McLaughlin, Lapierre, Johnson and Marcogliese2011; Besprozvannykh et al., Reference Besprozvannykh, Rozhkovan and Ermolenko2017; Pinto et al., Reference Pinto, Goncalves, López-Hernandez, Pulido-Murillo and Melo2018; Velázquez-Urrieta & Pérez-Ponce de León, Reference Velázquez-Urrieta and Pérez-Ponce de León2020, Reference Velázquez-Urrieta and Pérez-Ponce De León2021). Two novel investigations are worth mentioning. Selbach et al. (Reference Selbach, Soldanova, Feld, Kostadinova and Sures2020) and Duan et al. (Reference Duan, Al-Jubury, Kania and Buchmann2021) conducted comprehensive studies of cercarial diversity in freshwater ecosystems in Europe. The first one analysed 5347 snails of six species (from six lakes) and reported 36 species of trematodes, whereas the second analysed 5657 snails belonging to ten species (from 21 lakes) and reported 22 trematode species. Compared with our study, where we analysed 6434 molluscs belonging to two classes and 11 species, and reported 16 trematode species, it seems that species richness is lower in the tropical rainforest, where overall species diversity is much higher than that of temperate areas. Overall prevalence of infection is also contrasting. In our study, overall prevalence was very low – 1.8% – whereas in the studies by Selbach et al (Reference Selbach, Soldanova, Feld, Kostadinova and Sures2020) and Duan et al. (Reference Duan, Al-Jubury, Kania and Buchmann2021) prevalence was 19.6 and 12.6%, respectively. Irrespective of the causes that determine such difference between habitats, the two studies mentioned above were designed not just to investigate the trematode diversity, but to provide relevant data on transmission pathways as well as trophic interactions in the ecosystems. For instance, considering the uneven distribution and abundance of snails among waterbodies and after controlling for snail body size, Selbach et al. (Reference Selbach, Soldanova, Feld, Kostadinova and Sures2020) found no statistically significant differences in prevalence of infection between localities, seasons and years of sampling; these data allowed authors to show that trematode communities were temporarily and spatially stable. Ultimately, these authors discovered that the composition of snail populations was the main factor shaping the trematode community structure. This pattern was also discussed by Gordy et al. (Reference Gordy, Kish, Tarrabain and Hanington2016), who suggested that parasite diversity is not determined by snail host diversity but is determined by their species composition and abundance. Our results agree with such a pattern because only six of the 11 species of molluscs analysed (bivalves and gastropods) were infected, and one of the species, A. alleei, reached the highest trematode species richness, with five species. This cochliopid gastropod, along with B. heliophila, are the most abundant molluscs in each of the studied localities.
To the best of our knowledge, this is the first study to address the trematode larval diversity in molluscs of a tropical rainforest. Onaca et al. (Reference Onaca, Graca, Fabrin, Takemoto and Oliveira2020) characterized and identified the digenean larval stages in a neotropical floodplain in Brazil; however, they only analysed 26 specimens of one snail species, Aylacostoma chloroticum (Scott), and although they found an overall prevalence of infection of 73.08%, only three digenean species were reported. The approach we followed once again demonstrated to be a very precise way to advance our knowledge of trematode life cycles (Kudlai et al., Reference Kudlai, Stunaenas and Tkach2015). In addition, the approach is very useful for providing a better estimate of the trematode species diversity in the area, and for providing baseline data for the further monitoring of ecosystem health.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X21000407
Acknowledgements
This paper is part of the fulfilments of Y.V.U to complete her PhD program in the Posgrado en Ciencias Biológicas UNAM. Y.V.U. thanks CONACYT (Consejo Nacional de Ciencia y Tecnología) for granting a scholarship; we thank Berenit Mendoza, Laboratorio Nacional de Biodiversidad (LANABIO), for obtaining SEM photomicrographs, and Laura Márquez and Nelly López (LANABIO) for their help with the use of automatic sequencer. Special thanks are due to Rosamond Coates, Chief of the Estación de Biología Tropical Los Tuxtlas, for the facilities and permission to collect in Los Tuxtlas Biologial Station; we also thank Erli Velasco Sinaca for his help during field work. We sincerely thank two anonymous reviewers whose comments greatly improved the quality of our manuscript.
Financial support
This project was partially funded by the Consejo Nacional de Ciencia y Tecnología (CONACyT) A1-S-21694, and by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT-UNAM) IN212621 to G.P.P.L.
Conflicts of interest
None.
Ethical standards
Specimens were collected under the Cartilla Nacional de Colector Científico (FAUT 0057) issued by the Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT) to G.P.P.L., and under permission of the Estación de Biología Tropical de Los Tuxtlas.













