Introduction
Ovarian follicles are the fundamental units of the reproductive cycle and are responsible for gametogenesis and steroidogenesis. Oocytes are attached to and are surrounded by granulosa cells, which form the cumulus–oocyte complex (COC), and by theca cells (Morgan et al., Reference Morgan, Campbell, Allison, Murray and Spears2015; Zhang & Liu, Reference Zhang and Liu2015). The maturation process of the oocyte includes both the nuclear (meiosis) and cytoplasmic maturation processes (reorganization of organelles and cytoskeleton, transcription of RNAs, and translation of proteins related to embryonic development) (Ferreira et al., Reference Ferreira, Vireque, Adona, Meirelles, Ferriani and Navarro2009). The in vitro viability of COCs is essential for embryo development and is affected by the pubertal status of the female (Pawlak et al., Reference Pawlak, Warzych, Hryciuk and Lechniak2015) and FSH levels, which maintain the expression of BAX and BCL2 genes in the granulosa cells (Mani et al., Reference Mani, Fenwick, Cheng, Sharma, Singh and Wathes2010).
Steroidogenesis is one of the most important and well known factors involved in oocyte maturation both in vivo and in vitro. The intrafollicular concentrations of progesterone and 17β-estradiol are important to the oocyte's competence in generating embryos after in vitro fertilization (Aardema et al., Reference Aardema, Roelen, van Tol, Oei, Gadella and Vos2013) and affect the expression of zona pellucida proteins (Kempisty et al., Reference Kempisty, Woźna, Piotrowska, Bukowska, Jackowska, Antosik, Jaśkowski and Brüssow2012). Supplementing the COC culture medium with 17β-estradiol and progesterone improves the rate of embryos that develop to the morula stage, compared with medium containing only LH and FSH (Zheng et al., Reference Zheng, Si, Bavister, Yang, Ding and Ji2003). Higher in vitro concentrations of estradiol increased chromosomal aberrations of oocytes and diminished embryo development, whereas the addition of FSH reversed both effects (Beker et al., Reference Beker, Colenbrander and Bevers2002).
In addition, 17β-estradiol influences the viability and oxidative stress levels of cultured neurons by suppressing Bax and stimulating Bcl-2 expression levels (Li et al., Reference Li, Wang, Zhu, Feng, Wang, Shahzad, Hu, Mo, Du and Yu2013a; Li et al., Reference Li, Wu, Xue, Wang and Hou2013b). Phosphatidylinositol 3-kinase (PI3K) is essential for the viability effects of 17β-estradiol, and similarly, steroidogenesis is influenced by the levels of PI3K and the gonadotropins LH and FSH.
PI3K family proteins, which were discovered in 1980 (Liu et al., Reference Liu, Cheng, Roberts and Zhao2009), are kinases that phosphorylate 3′-hydroxyl inositol groups found in membrane lipid molecules known as phosphatidylinositols to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3) and other phosphatidylinositol phosphate molecules. The latter components recruit AKT/PKB to activate intracellular cascades that control growth, proliferation, survival/viability, metabolism, intracellular trafficking, and differentiation of cells (Hawkins et al., Reference Hawkins, Anderson, Davidson and Stephens2006; Engelman et al., Reference Engelman, Luo and Cantley2006; Yuan & Cantley, Reference Yuan and Cantley2008; Liu et al., Reference Liu, Cheng, Roberts and Zhao2009; Vanhaesebroeck et al., Reference Vanhaesebroeck, Guillermet-Guibert, Graupera and Bilanges2010).
PI3K proteins are classified into three different classes according to phosphorylation substrates and regions of sequence homology as follows (Hawkins et al., Reference Hawkins, Anderson, Davidson and Stephens2006; Engelman et al., Reference Engelman, Luo and Cantley2006): classes I (A and B), II and III; however, the last two classes are not completely understood. Class I is related to cell surface receptors; class II seems to be related to the internalization of receptors, cellular migration, glucose metabolism, exocytosis, apoptosis; and class III controls the intracellular transport of vesicles in the Golgi apparatus, cellular growth and autophagy (Engelman et al., Reference Engelman, Luo and Cantley2006; Vanhaesebroeck et al., Reference Vanhaesebroeck, Guillermet-Guibert, Graupera and Bilanges2010).
Class I proteins are clearly related to intracellular signalling, and the subtype IA proteins are activated by tyrosine kinase receptors and use p85 as the regulatory subunit, whereas subtype IB proteins are activated by G-protein-coupled receptors and do not use p85 (Vanhaesebroeck et al., Reference Vanhaesebroeck, Guillermet-Guibert, Graupera and Bilanges2010). The catalytic subunit can be p110α, p110β, p110γ or p110δ and promotes the activation of complex intracellular signalling using proteins including AKT/PKB, FOXO, PDK1, mTOR, MAPK and others (Engelman et al., Reference Engelman, Luo and Cantley2006; Vanhaesebroeck et al., Reference Vanhaesebroeck, Guillermet-Guibert, Graupera and Bilanges2010; Zheng et al., Reference Zheng, Nagaraju, Liu and Liu2012).
PI3K proteins participate in the activation and survival of primordial follicles and in the recruitment of follicles in ovarian cycles (Zheng et al., Reference Zheng, Nagaraju, Liu and Liu2012). FSH activates cAMP/PKA and PI3K, resulting in the increase of aromatase (CYP19) expression (Stocco, Reference Stocco2008). Aromatase converts androgens into estradiol, enhances the effects of FSH on COCs and progresses ovarian follicle development to a preovulatory stage (Stocco, Reference Stocco2008).
The effects of PI3K have been mostly evaluated in granulosa cells but not in bovine COCs. The aim of the present study was to analyze the in vitro effects of PI3K and its relationship to the effects of FSH on COC maturation, viability, steroidogenesis and embryo development. An inhibitor of PI3K, LY294002, was used to understand the intracellular effects of PI3K in vitro. It is noteworthy that oocytes were completely viable after culture, based on trypan blue tests, and viability, steroidogenesis and embryo development were significantly affected by PI3K.
Materials and methods
Bovine ovaries were obtained from a local slaughterhouse (Distrito Federal, Brazil) and transported to the laboratory in sterile saline solution (0.9% NaCl, w/v) at 35–37°C. The ovaries were rinsed twice in saline solution to remove the blood and were maintained at 35–37°C.
Follicles (1 to 8 mm in diameter) were aspirated with 21-gauge needles to obtain immature COCs. Ovarian follicles with clear fluid and well vascularized appearance were selected. The COCs were suspended in phosphate-buffered solution (PBS) (Invitrogen, Brazil) and BSA (Sigma, USA) under sterile conditions, and those characterized by compact cumulus cells and evenly granulated cytoplasm were selected (Viana et al., Reference Viana, De Almeida, De Moraes Ferreira, De Sa, De Carvalho Fernandes and De Pinho Marques2004).
After selection, COCs were cultured in defined, patented, serum free, basic maturation in vitro medium (MIVB) [applicant: Fundação Universidade de Brasilia]. MIVB is composed of a diluent alpha-MEM medium (Invitrogen-GIBCO, Brazil), HEPES (Sigma, USA), sodium bicarbonate, polyvinyl alcohol (PVA), non-essential amino acids, transferrin (Invitrogen-GIBCO, Brazil), 10–7 M androstenedione, and antibiotics, penicillin and streptomycin, supplemented with or without FSH 10 ng/mL (Sigma, USA). The inhibitor LY294002 (Sigma, USA) was used to determine the role of PI3K.
Experimental design
The experimental groups were MIVB with no FSH (0FSH), MIVB with no FSH supplemented with 10 μM or 100 μM of LY294002 (0FSH 10LY and 0FSH 100LY, respectively), MIVB with FSH 10 ng/ml (10FSH) and MIVB with FSH 10 ng/ml and 10 μM or 100 μM of LY294002 (10FSH 10LY and 10FSH 100LY, respectively). The doses of LY294002 were based on a study conducted by Hoshino et al. (Reference Hoshino, Yokoo, Yoshida, Sasada, Matsumoto and Sato2004). The group with the 10 μM dose was excluded from PCR assays and polar body extrusion rate determination because it did not demonstrate any effects.
All experiments procedures after in vitro maturation was based on the idea of comparing oocytes that matured in vitro with those ones that did not mature (extrude polar body) in terms of viability and gene expression.
In vitro maturation of COCs and the viability test
COCs (35–40 COCs/well) were cultured in 400 μl of medium for 22–25 h at 38.5°C, 5% CO2, and 95% humidity. The trypan blue exclusion test was performed to determine oocyte viability before and after culture, and no non-viable oocyte was found. Viability of COCs was also evaluated by embryo development in vitro.
The maturation rates were determined by the polar body extrusions after in vitro culture and were expressed as the average of percentages of matured oocytes per experiment.
In vitro fertilization and embryo culture
After culture, the COCs were fertilized according to the methods described by Mota et al. (Reference Mota, Oliveira e Silva, de Souza, Tuany, Pereira, Camargo and Rosa e Silva2015), and embryo culture was performed using SOF medium as described by Gulart (Reference Gulart2015).
Hormone level measurement
The activity of steroidogenesis was assessed by the measurement of hormones in the medium after culture. The culture medium of each well was collected and frozen at –20°C until hormone level analysis. Chemiluminescence was performed to measure 17β-estradiol (E2) and progesterone (P4) concentrations. The concentrations of both hormones were expressed as ng/ml, and the values were used to determine the E2/P4 ratios for each sample.
Isolation of cumulus cells and oocytes (polar body extrusion) after culture
After culture, the COCs were mechanically denuded and oocytes were isolated from cumulus cells. Isolated cumulus cells were centrifuged twice in PBS for 5 min at 6000 rpm. The oocytes and the pelleted cumulus cells were resuspended in PBS and TRIzol Plus (Life Technologies, Brazil) and stored at –80°C until RNA extraction.
The oocytes were divided into two categories: oocytes that matured and extruded polar bodies (progressed to metaphase II) and immature ones that did not extrude polar bodies [germinal vesicle/germinal vesicle breakdown (GV/GVBD), metaphase, anaphase or telophase I)]. The rate of polar body extrusion was correlated to 17β-estradiol (E2) and progesterone (P4) concentrations in the medium.
RNA extraction from cumulus cells
The TRIzol Plus (Life Technologies, Brazil) protocol was used for total RNA extraction. The isolated RNA was resuspended in RNase and pyrogen free water (Invitrogen) and stored at –80°C. RNA concentration and quality were measured using the NanoDrop 2000/2000c (Thermo Fisher Scientific) and 2100 Bioanalyzer (Agilent Technologies). The samples that presented NanoDrop A260/A280 ratios between 1.8 to 2.0 were used to synthesize cDNA samples.
cDNA synthesis and real-time PCR
The QuantiTect Reverse Transcription (Qiagen, USA) kit was used to perform cDNA synthesis based on the manufacturer's instructions, and the resulting samples were stored at –20°C.
Real-time PCR reactions were performed using the SYBR® Green Master Mix (Applied Biosystem, USA) with 50–100 ng/μl of cDNA, RNase and pyrogen free water (Invitrogen), and primer mixes that contained 100 nM forward and 100 nM reverse primers in the final volume of PCR reaction. The primers (Table 1) were designed based on the GenBank code and using the IDTSci Tools (Integrated DNA Technologies, USA), which included BLAST analysis. The real-time PCR conditions were previously standardized to guarantee high efficiency.
Table 1 Sequences and temperature melting of target bovine gene primers

FSHR: FSH receptor; LHR: LH receptor; CYP11A1: cytochrome P450, family 11-A1; CYP19A1: aromatase; HSD17B1: hydroxysteroid dehydrogenase 17β 1; CYC: cyclophilin A (peptidylprolyl isomerase A); BAX: BCL2 associated X; BCL-2: B cell CLL/lymphoma-2. Tm: melting temperature (°C).
The cycling conditions included the following steps: 95°C for 20 min, 45–50 cycles of 95°C for 3 s, and 60°C for 30 s, as described for the Fast SYBR® Green Master Mix. The melting curves confirmed that a single specific product was generated for each target gene, and no-template controls showed the absence of contaminants.
The relative quantification was calculated using the ΔΔCT method, and cyclophilin A (CYC) was chosen as the reference gene. The mean and standard deviation (SD) of CTs were 22.9 (±2.07, SD) for cumulus cells, and 32.68 (±2.86, SD) for oocytes. The calibrator group was the immature cumulus cells obtained from non-cultured COCs (GV). Three or four biological samples from each experimental group, and three or four replicates per biological sample, were analysed using PCR reactions.
A biological sample contained 15–25 oocytes or cumulus cells from 25 oocytes (each oocyte contains approximately 105 cumulus cells) (Calado et al., Reference Calado, Rocha, Colaço and Sousa2005). The COC pool was cultured for 24 h and after denudation, were grouped into oocytes that either extruded polar bodies and those did not. The cumulus cells were not separated based on the oocyte maturation state to avoid degeneration.
Statistical analysis
The chi-squared test was performed to analyze oocyte maturation (polar body extrusion). Two-way analysis of variance (ANOVA), followed by one-way ANOVA and the Bonferroni post hoc test were performed to analyze hormone measurements and E2/P4 ratios. Student's t-test was performed to determine the significant differences in the hormone measurements and E2/P4 ratios in the culture medium between the groups. The Kruskal–Wallis followed by Student–Newman–Keuls tests were performed to determine the differences in real-time PCR analysis among the groups.
The correlation between 17β-estradiol or progesterone and the nuclear maturation was evaluated by simple linear regression and Pearson's correlation. The differences were considered significant when the P-value was < 0.05.
Results
Medium supplemented with FSH significantly increased polar body extrusion compared with all groups, and PI3K inhibition blocked polar body extrusion of COCs cultured in 10FSH medium (Fig. 1). Interestingly, no degenerated COCs or oocytes were observed after culture. Only a few cumulus cells of one COC were degenerated and detached after culture (Figure S1). BAX and BCL2 gene expression levels were also tested in the cumulus cells and oocytes from cultured COCs and were differentially regulated based on the cell type (Figs 2 and 3).

Figure 1 Effects of FSH and PI3K inhibitor, LY294002, on polar body extrusion of oocytes isolated from bovine COCs that were matured in vitro. Oocytes per treatment 0FSH: 51 oocytes; 0FSH 100LY: 42 oocytes; 10FSH: 44 oocytes; 10FSH 100LY: 73 oocytes. The result was obtained from three independent experiments (replicates) of IVM. *Statistical difference from all groups (chi-squared test, P < 0.05). Values are expressed as averages of the maturation percentages of each experiment.
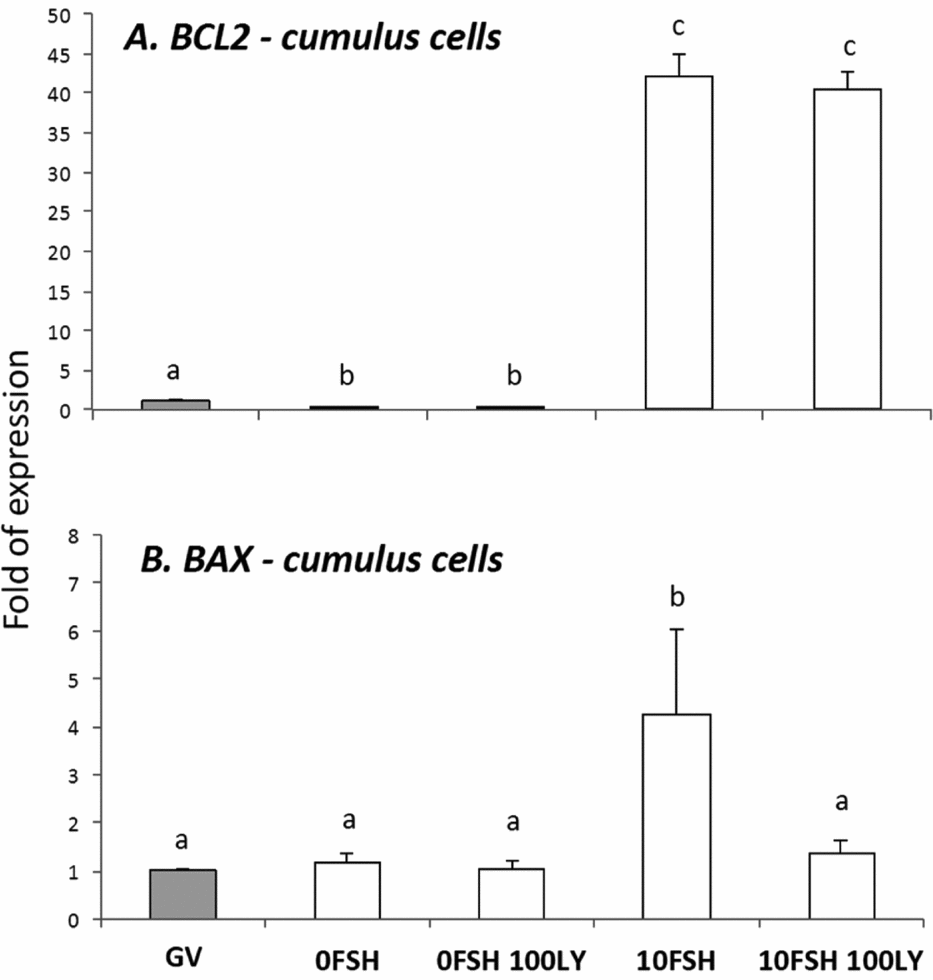
Figure 2 BAX and BCL2 gene expression levels in cumulus cells isolated from COCs after being cultured in a medium with or without FSH and PI3K inhibitor, LY294002. a,b,cDifferent letters indicate significant statistical differences among groups (P < 0.05).
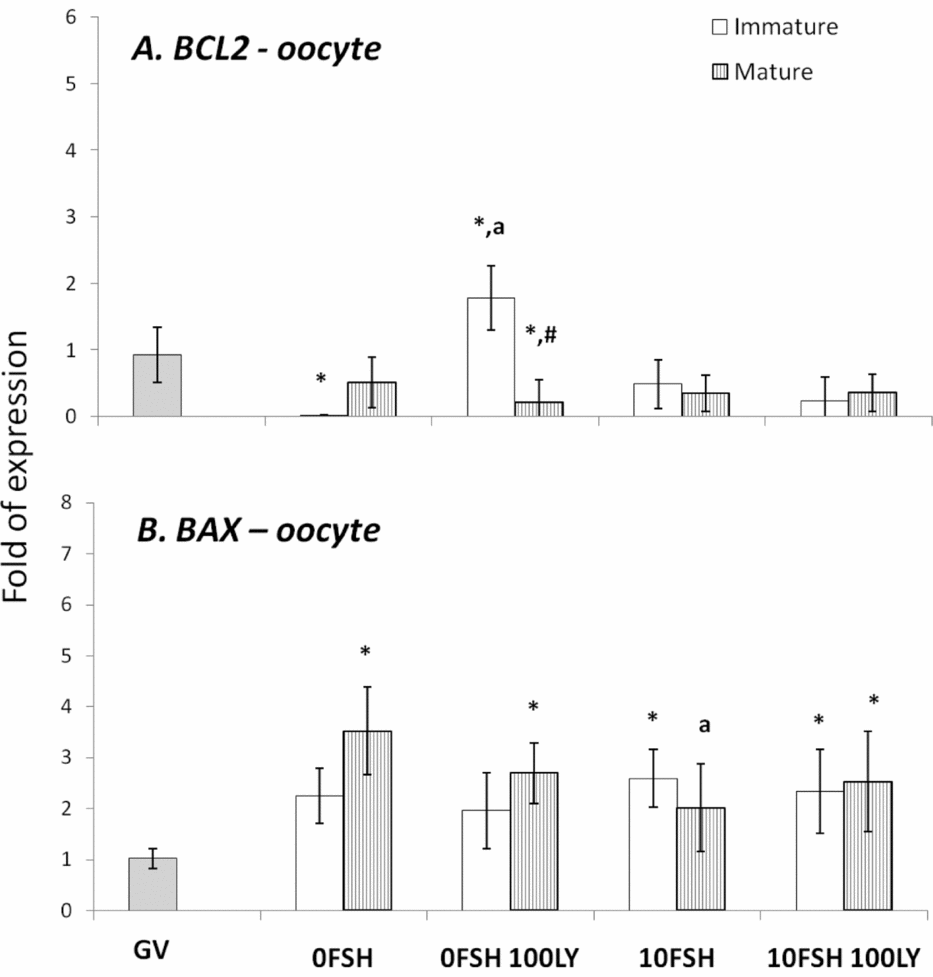
Figure 3 BAX and BCL2 gene expression levels in mature and immature oocytes isolated from COCs after being cultured in a medium with or without FSH and the PI3K inhibitor, LY294002. *Indicates significantly different from GV (P < 0.05). aIndicates significantly different from 0FSH group during the same cell status (P < 0.05). #Indicates significantly difference between immature and mature oocytes in the same experimental treatment.
In cumulus cells, both BCL2 and BAX expression levels were upregulated by FSH, however PI3K affected only BAX expression levels (Fig. 2). The oocytes expressed lower levels of BCL2 with the exception of immature oocytes cultured in the 0FSH 100LY medium (Fig. 3). Oocytes also expressed significantly lower levels of BCL2 mRNA compared with cumulus cells (data not shown; P < 0.05).
The BAX expression levels of oocytes (Fig. 3) are similar to those of cumulus cells (Fig. 2; P > 0.05); however, higher levels of mRNA were observed in both matured and immature oocytes after 24 h of culture (Fig. 3; P > 0.05).
In terms of steroidogenesis, FSH supplementation in the medium significantly enhanced the concentrations of E2 and P4; however, it is noteworthy that 0FSH medium was also able to sustain steroidogenesis (Table 2) in the presence of androstenedione. PI3K inhibition (100 μM of LY294002) significantly decreased E2 production and E2/P4 ratios in the 0FSH and 10FSH groups, whereas PI3K inhibition enhanced P4 production only in the 0FSH medium (Table 2). Progesterone did not demonstrate any relationship with polar body extrusion (R² = 0.009, P = 0.73); however, 17β-estradiol showed a significant correlation to nuclear maturation and polar body extrusion (R² = 0.564, P = 0.0020). The results demonstrate the essential role of PI3K in the steroidogenesis of COCs in vitro.
Table 2 PI3K and FSH effects in 17β-estradiol and progesterone concentrations and E2/P4 ratio of in vitro matured COCs

Significance expressed in the table is between 0FSH and 10FSH groups (Student's t-test).
a,bLetters indicates statistical difference between 0 μM, 10 μM and 100 μM LY294002 [one-way analysis of variance (ANOVA), P < 0.05].
Two-way ANOVA indicates statistical difference between the profile of 0FSH and 10FSH in each dose of inhibitor in 17β-estradiol (P = 0.0007) and progesterone (P < 0.0001), but not in E2/P4 ratio (P > 0.05).
Based on the activity of steroidogenic pathways as evaluated by E2 and P4 concentrations, the gene expression levels were evaluated after culture. The culture medium, absent of FSH (0FSH), enhanced the gene expression levels of LHR (Fig. 4 A), FSHR (Fig. 4 B), CYP11A1 (Fig. 5 A) and CYP19A1 (Fig. 5 B), but did not change the expression of HSD17B1, compared with the 10FSH group. In contrast, the 10FSH group presented lower levels of LHR, FSHR, CYP19A1 and HSD17B1 expression levels (Figs 4 and 5).

Figure 4 The roles of FSH and PI3K inhibition in the gene expression of the receptors of LH (LHR) and FSH (FSHR) in cumulus cells from bovine COCs cultured in vitro. a,b,c,dDifferent letters indicate significant statistical differences among groups (P < 0.05).

Figure 5 The roles of FSH and PI3K inhibition in the gene expression of the receptors of CYP11A1, CYP19A1 (aromatase) and HSD17B1 in cumulus cells from bovine COCs cultured in vitro. a,b,cDifferent letters indicate significant statistical differences among groups (P < 0.05).
PI3K inhibition decreased the expression of LHR (Fig. 4 A), FSHR (Fig. 4 B), CYP11A1 (Fig. 5 A), CYP19A1 (Fig. 5 B) and HSD17B1 (Fig. 5 C) when 0FSH medium was used. The FSH action was not affected by PI3K inhibition after 24 h of culture (Figs 4 and 5).
The in vitro rates of embryo development were similar to those described by Mota et al. (Reference Mota, Oliveira e Silva, de Souza, Tuany, Pereira, Camargo and Rosa e Silva2015), and no differences were observed between 0FSH and 0FSH 100LY groups (Fig. 6). 10FSH was able to increase embryo cleavage but not blastocyst development, and 10FSH 100LY decreased the cleavage and blastocyst production in vitro (Fig. 6). These results indicate the relevance of FSH to embryo development and show that PI3K alters FSH action in embryo development.
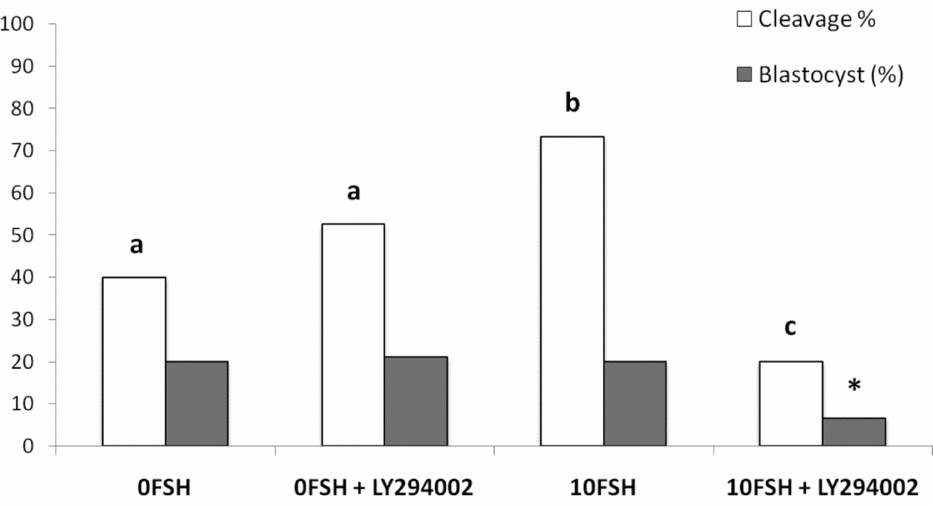
Figure 6 Cleavage and blastocyst rates of COCs after culture in the presence or absence of FSH and the PI3K inhibitor, LY294002. There were 168 oocytes, and 28 blastocyst embryos developed in vitro. The number of oocytes per replicate was 41 to 45 COCs, and the experiment was repeated three times. a,b,cDifferent letters indicate significant statistical differences among groups (P < 0.05). *Indicates significantly decreased compared with all groups.
Discussion
PI3K influenced nuclear maturation and blocks FSH effects in polar body extrusion, and PI3K inhibition did not degenerate oocytes after culture, despite changes in the gene expression levels of BAX and BCL2 in oocytes and cumulus cells. Steroidogenesis, mostly 17beta-estradiol, was also influenced by PI3K; it was not so clear, however, how the interactions between FSH and PI3K influenced gene expression. Similarly, estrogen was correlated to polar body extrusion, as well as PI3K and FSH. Interestingly, FSH increased embryo cleavage and the inhibition of PI3K severely decreased blastocyst development in vitro. To our knowledge, this is the first paper that describes the relevance of PI3K to bovine COCs in vitro.
Oocyte maturation corresponds to nuclear (polar body extrusion) and cytoplasmic maturation, which includes the production of RNAs and proteins for embryo development (Ferreira et al., Reference Ferreira, Vireque, Adona, Meirelles, Ferriani and Navarro2009). Polar body extrusion is strongly influenced by FSH (Mota et al., Reference Mota, Oliveira e Silva, de Souza, Tuany, Pereira, Camargo and Rosa e Silva2015) and PI3K. The F-actin in oocytes is dependent on PtdIns(3,4,5)P3, which is produced by proteins such as PI3K. The inhibition of PtdIns production modifies the migration of the meiotic fuse to the peripheric oolemma (Zheng et al., Reference Zheng, Baibakov, Wang and Dean2013) and could potentially explain the inhibition of polar body extrusion by LY294002 even in the presence of FSH in the medium.
Despite the nuclear maturation results, viability was not influenced by PI3K in vitro as shown by the trypan blue test. The PI3K/AKT proteins control apoptosis, and LY294002 increased the number of dead cumulus cells from porcine COCs cultured with FSH (Shimada et al., Reference Shimada, Ito, Yamashita, Okazaki and Isobe2003). In addition, BAX and BCL2 levels of the oocytes were not strongly modulated by PI3K, and the cumulus cells were very responsive to FSH and PI3K. As previously described, the BAX and BCL2 levels of the granulosa cells are controlled and stimulated by FSH, and only BAX levels respond to PI3K (Mani et al., Reference Mani, Fenwick, Cheng, Sharma, Singh and Wathes2010); this is similar to the results presented here.
The viability of cultured cells is clearly influenced by 17β-estradiol (Li et al., Reference Li, Wang, Zhu, Feng, Wang, Shahzad, Hu, Mo, Du and Yu2013a, b). Higher concentrations of 17β-estradiol in the COC culture medium (10–4 mol/l) significantly increased oocyte diameter, nuclear maturation and cumulus expansion in porcine oocytes (Kubo et al., Reference Kubo, Cayo-Colca and Miyano2015); however, higher rates of chromosomal aberrations were identified in bovine oocytes (Beker et al., Reference Beker, Colenbrander and Bevers2002). Progesterone and estradiol also influenced the expression of zona pellucida glycoproteins in canine oocytes (Kempisty et al., Reference Kempisty, Woźna, Piotrowska, Bukowska, Jackowska, Antosik, Jaśkowski and Brüssow2012), demonstrating the relevance of progesterone and estradiol to the fertilization process.
The 0FSH medium was able to sustain steroidogenesis based on androstenedione precursor and, as expected, the addition of FSH enhanced its ability. The patented MIV B medium is different from MIV C due to its lack of hormones as shown in a previous analysis (Vasconcelos et al., Reference Vasconcelos, Salles, Oliveira e Silva, Gulart, Souza, Torres, Bocca and Rosa e Silva2013). MIV C was described as a pro-estrogenic medium in a study in which the wall sections of bovine ovarian follicles were cultured in vitro with medium containing no FSH and supplemented with androstenedione and other hormones (Vasconcelos et al., Reference Vasconcelos, Salles, Oliveira e Silva, Gulart, Souza, Torres, Bocca and Rosa e Silva2013). The MIV B medium is also estrogenic and sustains COCs in vitro even when FSH is absent (E2:P4 ratio is approximately 1.0).
FSH is a well known hormone related to follicle steroidogenesis (Gutiérrez et al., Reference Gutiérrez, Campbell and Webb1997; Silva et al., Reference Silva, Hamel, Sahmi and Price2006; Zheng et al., Reference Zheng, Price, Tremblay, Lussier and Carrière2008), and cumulus cells are considered more progesteronic than mural granulosa cells when exposed to higher concentrations of FSH (Armstrong et al., Reference Armstrong, Xia, de Gannes, Tekpetey and Khamsi1996). Supplementation with 10 ng/ml of FSH was able to maintain higher levels of 17β-estradiol compared with progesterone (Zheng et al., Reference Zheng, Price, Tremblay, Lussier and Carrière2008). MIV B+FSH sustained the E2:P4 ratio at approximately 1.0, similar to the 0FSH medium; however, the absolute levels of both steroid hormones were higher in the FSH medium as expected.
The mRNA expression levels were differently regulated based on the presence or absence of FSH. Exposure to FSH for 24 h decreased the expression levels of steroidogenic enzymes (with exception of CYP11A1) and gonadotropin receptors, despite the enhancement of steroidogenic activity, which was demonstrated by E2 and P4 concentrations. Culture medium that did not contain FSH sustained higher levels of LHR, FSHR, CYP11A1, CYP19A1 and HSD17B1 expression.
Steroid production depends on gonadotropins and enzymes, and previous studies have demonstrated that FSH controls the expression levels of CYP11A1, CYP19A1, HSD17B1, LHR and FSHR in COCs and granulosa cells (Silva & Price, Reference Silva and Price2002; Calder et al., Reference Calder, Caveney, Smith and Watson2003; Sahmi et al., Reference Sahmi, Nicola and Price2006). However, the gene expression levels of gonadotropins and enzymes are not directly related to hormone production. The lower levels of expression can be related to the downregulation of FSH receptors after 24 h of exposure to hormones (Houde et al., Reference Houde, Lambert, Saumande, Silversides and Lussier1994) or the mRNA stability during culture (Sahmi et al., Reference Sahmi, Nicola and Price2006; Payne, Reference Payne2015). Cultured COCs showed decreased expression levels of the FSH receptor, whereas the LH receptor levels increased (Calder et al., Reference Calder, Caveney, Smith and Watson2003; Salhab et al., Reference Salhab, Tosca, Cabau, Papillier, Perreau, Dupont, Mermillod and Uzbekova2011). The levels of CYP11A1 and CYP19A1 decreased during culture (Salhab et al., Reference Salhab, Tosca, Cabau, Papillier, Perreau, Dupont, Mermillod and Uzbekova2011), and HSD17B1 expression in COCs has not been fully explored. In bovine granulosa cells, mRNA stability was observed for 3 h for CYP19A1 mRNA and for 12 h for CYP11A1 and HSD17B1 mRNAs (Sahmi et al., Reference Sahmi, Nicola and Price2006).
In terms of intracellular signalling, PI3K has been extensively investigated in cancer cells, and its mutations are frequently identified in cancer cell lines and somatic cells (Yuan and Cantley, Reference Yuan and Cantley2008; Liu et al., Reference Liu, Cheng, Roberts and Zhao2009), making this protein a pharmacological target (Liu et al., Reference Liu, Cheng, Roberts and Zhao2009, Reference Liu, Cheng, Santiago, Raeder, Zhang, Isabella, Yang, Semaan, Chen, Fox, Gray, Monahan, Schlegel, Beroukhim, Mills and Zhao2011). PI3K is one of the most relevant proteins that controls steroidogenesis (Moore et al., Reference Moore, Otsuka and Shimasaki2001; Shimada et al., Reference Shimada, Ito, Yamashita, Okazaki and Isobe2003; Yu et al., Reference Yu, Han, Yang, Jin, Hu and Liu2005; Su et al., Reference Su, Nyegaard, Overgaard, Qiao and Giudice2006; Ebeling et al., Reference Ebeling, Töpfer and Meinecke2011) and has defined actions in the activation and survival of primordial follicles, and in the recruitment of follicles (Zheng et al., Reference Zheng, Nagaraju, Liu and Liu2012).
PI3K controls steroidogenesis, an effect that has been observed by using 100 μM of its inhibitor, LY294002, a dose used by Hoshino et al. (Reference Hoshino, Yokoo, Yoshida, Sasada, Matsumoto and Sato2004). The steroid concentrations in the culture medium after 24 h of culture indicate the relevance of PI3K, which decreases 17β-estradiol and the E2:P4 ratio in the presence or absence of FSH. In the absence of FSH, progesterone production was significantly enhanced after PI3K inhibition. LY294002 caused a significant decrease in LHR, FSHR, CYP11A1, CYP19A1 and HSD17B1 expression levels in 0FSH medium but demonstrated no effects in 10FSH.
Previous results have indicated that, in the presence of FSH, progesterone production is stimulated by LY294002 in cumulus cells (Shimada et al., Reference Shimada, Ito, Yamashita, Okazaki and Isobe2003) and MAPK (protein controlled by PI3K) is inhibited, which indicates that progesterone levels decrease and estradiol levels increase in cumulus and granulosa cells (Moore et al., Reference Moore, Otsuka and Shimasaki2001; Yu et al., Reference Yu, Han, Yang, Jin, Hu and Liu2005; Su et al., Reference Su, Nyegaard, Overgaard, Qiao and Giudice2006; Ebeling et al., Reference Ebeling, Töpfer and Meinecke2011). Our data demonstrated that steroidogenesis is dependent on, but not exclusively so, PI3K action because steroidogenesis occurs even in the presence of LY294002.
Previous results have shown that PI3K or MAPK inhibition, in the presence of FSH, resulted in differing effects on CYP11A1 and CYP19A1 gene expression levels in COCs and granulosa cells (Moore et al., Reference Moore, Otsuka and Shimasaki2001; Su et al., Reference Su, Nyegaard, Overgaard, Qiao and Giudice2006; Ebeling et al., Reference Ebeling, Töpfer and Meinecke2011), and no studies have reported the effects on FSHR, LHR and HSD17B1 gene expression levels. The lack of additional information about the relevance of PI3K to steroidogenic gene expression in COCs cultured in vitro makes the current study an important contribution to the understanding of oocyte maturation. It is noteworthy that steroidogenesis inhibitors (AGT) reduce the production of estradiol and progesterone and, as expected, impair the in vitro maturation of COCs. Supplementation of both hormones did not recover maturation rates, which indicates that other relevant factors are involved (Wang et al., Reference Wang, Isobe, Kumamoto, Yamashiro, Yamashita and Terada2006).
In terms of in vitro embryo development, COCs cultured in the 0FSH medium developed to the blastocyst stage independently of the presence of LY294002, in accordance with the observations of Mota et al (Reference Mota, Oliveira e Silva, de Souza, Tuany, Pereira, Camargo and Rosa e Silva2015). The 10FSH medium increased embryo cleavage but not blastocyst development, and COCs matured in vitro with LY294002 decreased both parameters. The inhibition of PI3K blocked the essential effects of FSH in oocyte competence, including embryo development in vitro, despite the expression levels of BAX and BCL2. The inability to extrude polar bodies can be associated with low-quality embryos, lower in vitro development and genomic aberrations (Somfai et al., Reference Somfai, Kikuchi, Medvedev, Onishi, Iwamoto, Fuchimoto, Ozawa, Noguchi, Kaneko, Ohnuma, Sato and Nagai2005). The concentration of 17β-estradiol in the culture medium was also lower in the group 10FSH 100LY, indicating some possible correlation.
In conclusion, the relevance of FSH and PI3K to COC maturation is essential as observed by polar body extrusions, steroidogenesis and embryo development in vitro, although the gene expression levels were not directly related to oocyte competence and blastocyst rates.
Acknowledgements
The authors would like to thank CNPq and FAP-DF (193.000.577/2009) for grants. We would also like to thank Ponte Alta abattoir for supplying the ovaries and Isabela Bessa, Silene Silva, Raul Holanda and Daniele Cristiane for their collaboration on the experiments and projects.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0967199417000703
Conflicts of interest
The authors claim that there are no conflicts of interest.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Apoio à Pesquisa do Distrito Federal (FAP-DF) (193.000.577/2009), Brazil.
Danielle Kaiser de Souza was the recipient of a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) fellowship from the Medical Sciences Program/University of Brasilia.
Supporting information
Additional Supporting information is available online at the publisher's website.
Figure S1 Viability test of COC after culture in the presence or absence of FSH and the PI3K inhibitor, LY294002.












