Introduction
The developmental origin of health and disease (DOHaD) hypothesis suggests that the environment a fetus experiences in utero can impact that individual’s health for the rest of their lives. Reference Barker1 Barker observed that fetuses born very small had increased risk of cardiovascular disease as adults. Reference Barker2 Maternal conditions such as obesity and diabetes can also affect the fetus’s development, thus increasing the risk for later-life disease. Reference Oken and Gillman3,Reference Karter, Rowell and Ackerson4 These examples demonstrate that individuals who have experienced negative in utero programming through maternal, placental, or fetal causes experience altered development that increases their risk for later-life health complications, many of which are metabolic in nature. Cardiovascular disease, obesity, and diabetes affect increasingly large portions of our population. Understanding the early-life programming that contributes to them may help reduce the burden of these health concerns for future generations. In this review, we will focus on the role of in utero magnetic resonance imaging (MRI) in the study of cardiovascular and metabolic consequences of the DOHaD relationship through fetal, placental, and maternal metabolic perturbations.
MRI was developed in the 1970s and is used to visualize the internal anatomy and assess the function of the human body. Reference Mansfield and Maudsley5 In the 1980s, MRI was used to visualize the pregnant anatomy, offering a new modality to visualize the fetus and placenta. Reference Smith, Adam and Phillips6 A precautionary approach to safety limited the widespread adoption of MRI in pregnancy for many years. Current use of in utero MRI follows guidelines such as those set out by the American College of Radiology. 7 Recent safety studies have not found an increase in adverse outcomes during pregnancy and early childhood after in utero exposure to MRI if contrast agents are not used. Reference Ray, Vermeulen, Bharatha, Montanera and Park8–Reference Bulas and Egloff12 The major concerns related to MRI in pregnancy include heating of the fetus and placenta, acoustic noise damage to fetal and maternal hearing, and potential teratogenicity of magnetic fields. A more in-depth look into these concerns is presented in other review papers. Reference Lum and Tsiouris13,Reference De Wilde, Rivers and Price14
The use of in utero MRI is increasing in both research and clinical practice as it offers many advantages over other imaging modalities. Reference Glenn15 MRI does not use ionizing radiation and is noninvasive, making it safe/ideal to use in studying a vulnerable population. Reference Stecco, Saponaro and Carriero10 It is a modality with a large field-of-view, allowing visualization of the entire uterus in an image volume. Reference Levine16 MRI provides excellent soft-tissue contrast and is multiparametric, enabling multiple contrast sources in a single examination. Reference Frates, Kumar, Benson, Ward and Tempany17,Reference Meyer-Wittkopf, Cook, McLennan, Summers, Sharland and Maxwell18 Some of the MRI contrasts allow us to measure information about function in addition to structure, permitting assessment of oxygenation, metabolism, and perfusion, to name a few. Fast 2D imaging, such as single-shot spin-echo sequences, is routinely used to assess fetal and organ growth and development, especially in the brain. Diffusion imaging is frequently used to investigate pathological placental invasion Reference Dekan, Linduska, Kasprian and Prayer19,Reference Aughwane, Ingram, Johnstone, Salomon, David and Melbourne20 and has been used to measure lung maturity. Reference Moore, Strachan, Tyler, Baker and Gowland21,Reference Balassy, Kasprian and Brugger22 For more information on the basic MRI principles and essential topics related to fetal MRI, please refer to the recent review paper by Aertsen et al. Reference Aertsen, Diogo, Dymarkowski, Deprest and Prayer23
In comparison, the use of positron-emission tomography (PET) and x-ray computed tomography (CT) in pregnant humans is uncommon due to the risks associated with ionizing radiation. Reference Sreetharan, Thome and Tharmalingam24 Nuclear medicine, including PET, is only performed on pregnant patients for diagnosis or therapy of life-threatening conditions. Reference Stabin25 While CT remains an essential tool in diagnostics regardless of pregnancy status, it is not commonly used in pregnancy research. Reference Goldberg-Stein, Liu, Hahn and Lee26 Neither of these modalities are used in prospective pregnancy research, as there is no clinical benefit to the patient to outweigh the risk of the ionizing radiation delivered through imaging. Ultrasound (US) is frequently used in pregnancy to assess structure and limited functional parameters. 27 Its relatively small field-of-view and typical 2D image presentation make visualization of the entire fetus difficult late in gestation. Reference Elliott28 US is also relatively insensitive to metabolism and oxygenation.
MRI does have limitations such as accessibility Reference Iron, Przybysz and Laupacis29 and cost. Reference Sarracanie, LaPierre, Salameh, Waddington, Witzel and Rosen30 These barriers include health centers without access to advanced MRI techniques Reference Iron, Przybysz and Laupacis29 or expertise to use them in utero. Furthermore, MRI is a relatively slow imaging technique and is therefore sensitive to maternal and fetal motion, the latter of which is random and unpredictable. Reference Quinn, Hubbard and Adzick31 When investigating a developing fetus’s small anatomy, partial volumes Reference Kneeland, Shimakawa and Wehrli32,Reference Simmons, Tofts, Barker and Arridge33 and spatial resolution Reference Van Reeth, Tham, Tan and Poh34 can be limiting. Additionally, the physical configuration of the MRI system can limit the size of the patient that can be imaged, particularly in the late second and third trimesters. The increasing availability of larger bore MRI systems (70 cm bore) is alleviating this issue. Despite these limitations, in utero MRI provides us with a wealth of knowledge about fetal growth, development, and programming not previously available.
This review will describe different MRI techniques used to study the cardiovascular and metabolic consequences of DOHaD (Table 1). We will discuss the investigation of fetal growth and organ development, body composition and adipose tissue, oxygenation and oximetry, placental microstructure, diffusion, perfusion and flow, and metabolism using in utero MRI.
Table 1. Summary of different MRI techniques and their in utero application for studying the metabolic and cardiovascular consequences of DOHaD in humans

Assessment of fetal growth and organ development
Fetal growth assessment is regularly used to identify fetuses growing insufficiently (fetal growth restriction, FGR) or excessively (macrosomia), both of which are associated with the later-life metabolic consequences of DOHaD. MRI can monitor fetal growth through volumetric measurements, which have been shown to be superior to US estimated fetal weight. Reference Kadji, Cannie and Resta35,Reference Carlin, Kadji, Cannie, Resta, Kang and Jani36 The excellent soft-tissue contrast provided by MRI also allows for the measurement of organ growth, including liver, kidneys, brain, lungs, heart, and placenta. Reference Prayer and Brugger37 The assessment of differential organ growth is useful for identifying altered growth distributions such as asymmetric FGR. MRI sequence parameters are chosen to optimize the images for an organ or region of interest, and in some cases, specialized acquisitions and reconstructions are required. Most notably, the fetal heart requires dedicated MRI sequences due to the motion through the cardiac cycle. Recent fetal cardiac MRI techniques are discussed in a review by Macgowan et al. Reference Roy, van Amerom, Marini, Seed and Macgowan38 These techniques could be applied to study cardiac remodeling in response to an adverse in utero environment as they offer excellent structural views of the fetal heart through the entire cardiac cycle. While the use of MRI for fetal organ assessment has been focused on understanding normal development or assessing congenital malformations, the knowledge gained will help future studies on the cardiovascular and metabolic consequences of the DOHaD hypothesis.
Adipose tissue and body composition
The earliest reports of MR images in pregnancy include a description of visible fetal adipose tissue. Reference Smith, Adam and Phillips6,Reference Smith, MacLennan, Abramovich, MacGilivray and Hutchison39 Today, the ability to image adipose tissue, including that of the fetus, has far surpassed these early descriptions. As a result, a review of current techniques to image adipose tissue shows how refined and varied these newer tools have become. Reference Ma40,Reference Lemos and Gallagher41
Fetal adipose tissue can be used to assess developmental programming’s effects because it reflects the fetus’s energy deposition through pregnancy. Reference Ornoy42 For instance, fetuses of mothers with diabetes have an increased risk of being born with macrosomia, often due to an increase in offspring adipose tissue. Reference Catalano, Thomas, Huston-Presley and Amini43 Changes in adipose tissue may be an early indicator of an altered fetal metabolism that could result in the development of metabolic syndrome later in life. Through fetal MRI, Anblagan et al. observed that fetuses of mothers with pre-existing diabetes had a greater adipose tissue volume than controls. Reference Anblagan, Deshpande and Jones44 Using fat-only images at 34 weeks gestation, they found the total adipose tissue volume was increased and identified intra-abdominal adipose tissue more often in the fetuses of diabetic mothers. Reference Anblagan, Deshpande and Jones44 Furthermore, using fat-only MR images, Berger-Kulemann et al. found that the fetuses of mothers with well-controlled diabetes did not have thicker subcutaneous adipose tissue than controls and attributed this to the strict control of the maternal glucose metabolism. Reference Berger-Kulemann, Brugger and Michael45 This finding suggests that careful control of the mother’s metabolism may minimize the effects of metabolic diseases such as diabetes on the fetus’s prenatal programming.
MRI is also capable of measuring the amount of lipid within a tissue using a method known as chemical shift encoded (CSE) MRI (see Fig. 1 for example images). CSE-MRI exploits the different resonant frequencies of lipids and water to create quantitative images of lipid or water content of tissues. Reference Reeder, Hu and Sirlin46 Giza et al. have used CSE-MRI in the fetus to show that fetal adipose tissue has an increasing proportion of lipid as the pregnancy progresses. Reference Giza, Olmstead and McCooeye47 Furthermore, this technique has been applied to study a maternal high-fat diet’s effect on fetal adipose tissue development in guinea pigs. Reference Sinclair, Friesen-Waldner and McCurdy48 Sinclair et al. found that mothers exposed to a lifelong high-fat diet produced fetuses with increased lipid deposited in the adipose tissue and liver compared to those on a control diet. Reference Sinclair, Friesen-Waldner and McCurdy48 These examples provide evidence that maternal metabolism can influence fetal metabolism to the extent that alterations in fetal adipose tissue development and lipid deposition are detectable with MRI, which may give early insight into the programming of the metabolic consequences of DOHaD.
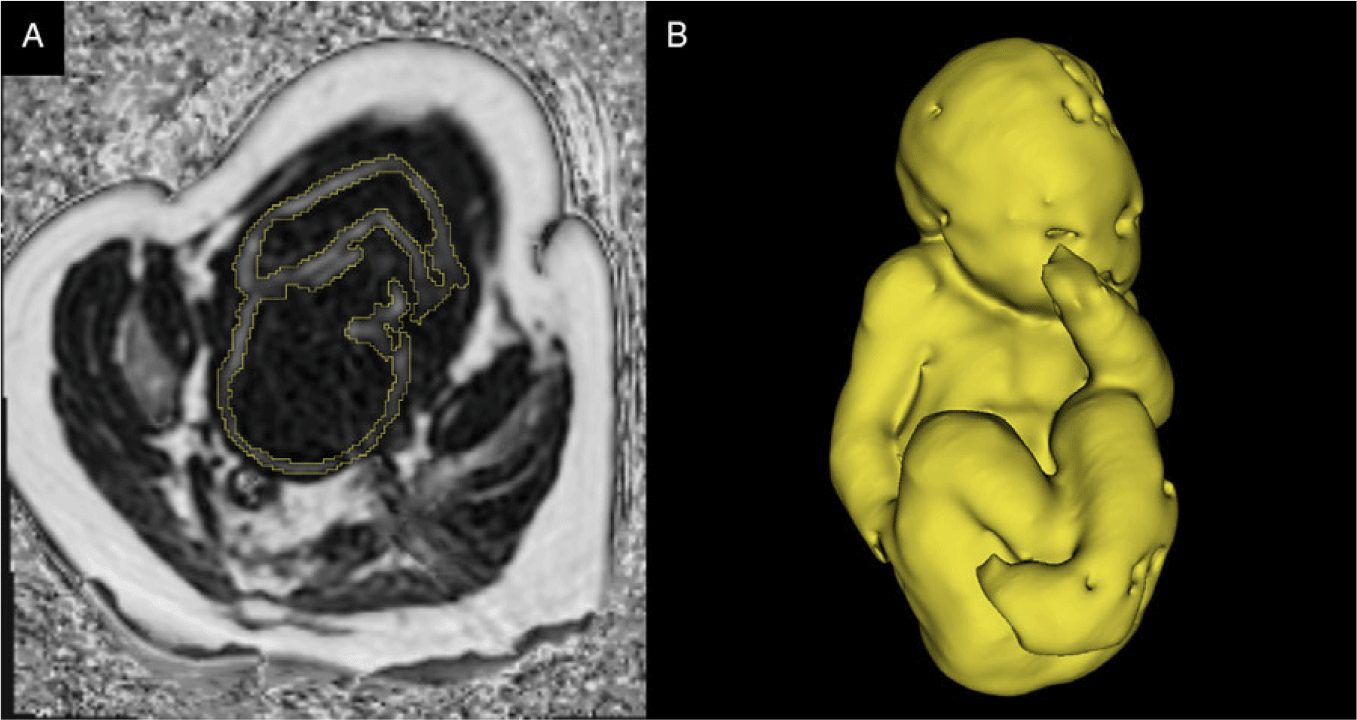
Fig. 1. (A) A single slice of 3D CSE-MRI image of third-trimester pregnancy. Bright pixels represent areas with high lipid content, and dark pixels represent areas of low lipid content. The segmentation of fetal adipose tissue is shown in yellow. (B) 3D rendering of segmented fetal adipose tissue. Image courtesy of the Pregnancy Research Group.
Fetal motion is a significant obstacle for fetal MRI, including MRI of fetal adipose tissue. In the future, application of motion-resistant techniques, such as radial CSE-MRI, Reference Benkert, Feng, Sodickson, Chandarana and Block49 to image the fetus should mitigate the problem of motion. CSE-MRI can also assess fatty acid saturation to determine the proportions of saturated, mono-unsaturated, and poly-unsaturated fatty acids in tissue. Reference Bydder, Girard and Hamilton50 This technique could give more insight into both maternal and fetal lipid metabolism.
After measuring fetal adipose tissue volume, fetal body composition can be determined by combining the adipose tissue measure with total fetal volume. Anblagan et al. used this idea to create a formula for estimating fetal weight using the different densities for fat mass and fat-free mass. Reference Anblagan, Deshpande and Jones44 This technique could be extended to include a bone measurement using any MRI technique specialized for imaging bone Reference Werner, Lopes Dos Santos, Ribeiro, Belmonte, Daltro and Araujo Junior51 to determine the three main body composition compartments: bone mass, fat mass, and fat-free mass. When applied to the fetus, this may allow researchers to study the effects of metabolism changes on any compartments of the developing body composition.
Fetal and placental oximetry
One of the placenta’s many functions is to provide the fetus with an adequate supply of oxygen. Reference Gude, Roberts, Kalionis and King52 However, if the placenta is impaired and unable to provide the fetus with adequate oxygen, the fetus is at risk for severe health conditions such as FGR. Reference Cetin and Alvino53,Reference Pardi, Marconi and Cetin54 Although techniques exist to assess the placenta’s oxygen exchange, they are either invasive Reference Liao, Wei, Li, Li, Li and Li55 or based on indirect measurements. Reference Madazli, Somunkiran, Calay, Ilvan and Aksu56–Reference Baschat58 Fortunately, MRI provides noninvasive techniques that can be used to accurately measure changes in fetal and placental oxygenation.
MRI is sensitive to blood-oxygen content because of the difference in magnetic susceptibility between oxygenated and deoxygenated hemoglobin. Reference Pauling and Coryell59 Two techniques that utilize this difference in magnetic properties are blood-oxygen-level-dependent (BOLD) MRI and vascular relaxometry.
BOLD imaging, often used in the brain, is the MR contrast mechanism used in functional MRI (fMRI) to visualize blood oxygenation changes related to neuronal activation. Reference Logothetis60 Deoxyhemoglobin results in a decrease in the BOLD-fMRI signal, while oxyhemoglobin results in an increase, Reference Ogawa and Lee61 allowing one to visualize the oxygen saturation change.
Numerous publications have focused on the use of BOLD-fMRI for assessing oxygenation in the placenta and other fetal organs in both animals and humans. It has been used to assess oxygen saturation change in the placenta and fetal organs in lambs under maternal hypoxia. Reference Wedegartner, Tchirikov and Schafer62–Reference Sorensen, Holm and Pedersen64 Sorensen et al. further investigated fetal liver oxygenation change in lambs, finding an oxygen saturation change in response to maternal hypoxia to be more pronounced in the right side of the fetal liver, which could be due to increased venosus shunting. Reference Tchirikov, Schroder and Hecher65 Interestingly, unlike the placenta and other fetal organs, the fetal brain did not experience a change in the BOLD-fMRI signal when placed under hypoxic, normoxic, or hyperoxic conditions in fetal lambs. Reference Sorensen, Pedersen, Tietze, Ottosen, Duus and Uldbjerg66 This finding suggests a brain sparing mechanism Reference Cohn, Sacks, Heymann and Rudolph67–Reference Pearce69 that was also observed in fetal mice. Reference Cahill, Zhou, Seed, Macgowan and Sled70 In contrast, Wedegärtner et al. found a decrease in the BOLD-fMRI signal in fetal lamb brain, heart, and liver during maternal hypoxia, though the decrease in signal was significantly smaller in the brain compared to the other two organs. Reference Wedegärtner, Tchirikov, Koch, Adam and Schröder71 BOLD-fMRI was also used to show a difference in response to maternal hyperoxygenation in growth-restricted fetal rats as they experienced a smaller increase in fetal tissue oxygenation compared to control fetal rats. Reference Aimot-Macron, Salomon and Deloison72
In human studies, BOLD-fMRI has been used to investigate placental and fetal oxygenation primarily during maternal hyperoxia, a treatment implemented for increasing fetal oxygenation by providing oxygen to the mother. Reference Young, Popat, Luther, Scott and Writer73,Reference Willcourt, King and Queenan74 During maternal hyperoxia, the oxygenation of the placenta and the fetal liver, fetal spleen, and fetal kidney was found to increase. Reference Sorensen, Peters, Frund, Lingman, Christiansen and Uldbjerg75,Reference Sorensen, Peters and Simonsen76 The brain sparing mechanism observed in fetal rats Reference Cahill, Zhou, Seed, Macgowan and Sled70 has also been seen in humans as fetal brain oxygenation was not found to increase in response to maternal hyperoxia, in contrast to other fetal tissues. Reference Sorensen, Peters and Simonsen76,Reference Huen, Morris, Wright, Sibley, Naish and Johnstone77 Luo et al. further explored the utility of the BOLD-fMRI signal using placental time-to-plateau maps to assess placental oxygen transport in monozygotic twin pairs to encapsulate regional variations in placental function. This study found that in growth-restricted twins, the smaller twin needed more time to reach a hyperoxic steady state. Reference Luo, Abaci Turk and Bibbo78 In summary, BOLD-fMRI has been used to successfully assess placental and tissue oximetry changes, which could provide information regarding the consequences of placental dysfunction in DOHaD.
BOLD-fMRI only provides relative oxygenation values rather than a quantitative value or quantitative change for blood volume or oxygen saturation. Reference Ingram, Morris, Naish, Myers and Johnstone99 Despite these limitations, it has the potential to be useful in many clinical applications. The next step for BOLD-fMRI would be assessing fetal response to maternal hyperoxia in cases of severely impaired placental function to better identify fetal nonresponsiveness, which is a predictor of adverse neonatal outcome. Reference Sorensen, Peters and Simonsen76
Vascular relaxometry is the measurement of the MR relaxation times (T1, T2, and T2*) of blood. Reference Cistola and Robinson79 Due to the difference in deoxyhemoglobin and oxyhemoglobin’s magnetic properties, the relaxation parameters of blood are sensitive to oxygen content. Reference Ogawa and Lee61 By quantifying these relaxation times, fetal vascular properties, such as blood hematocrit and oxygen saturation, can be determined. Reference Thulborn, Waterton, Matthews and Radda80–Reference Silvennoinen, Kettunen and Kauppinen83 T1 and T2 relaxation times of fetal blood have been quantified as a function of blood hematocrit and oxygen saturation at 3.0 Tesla. Reference Portnoy, Milligan, Seed, Sled and Macgowan84 Human umbilical cord blood’s relaxation times have also been quantified Reference Portnoy, Osmond, Zhu, Seed, Sled and Macgowan85 and used to create models that estimate blood hematocrit and oxygen saturation. Reference Portnoy, Seed, Sled and Macgowan86 Zhu et al. found the umbilical vein’s T2 relaxation time to be a useful marker for assessing response to hypoxia in growth-restricted fetuses. Reference Zhu, Milligan and Keating87 Another methodology that has been successful in assessing blood oxygenation is susceptibility-weighted imaging. The referenced studies have successfully used the principles of MRI susceptometry to evaluate fetal cerebral venous blood oxygenation saturation. Reference Yadav, Buch and Krishnamurthy88,Reference Neelavalli, Jella and Krishnamurthy89 Furthermore, Yadav et al. showed a decrease in cerebral blood oxygenation with increasing gestation in second- and third-trimester fetuses, Reference Yadav, Krishnamurthy and Buch90 most likely due to the marginal decrease in the umbilical venosus blood oxygenation and increased metabolic demands of the fetus during the investigated gestational period. Reference Schröter, Chaoui, Glatzel and Bollmann91,Reference Burton and Jaunaiux92
Tissue relaxometry has also been investigated, particularly in the placenta. Reference Gowland, Freeman and Issa93–Reference Sinding, Peters and Frokjaer96 In the case of placental dysfunction, T1, T2, and T2* were found to be lower than in gestational age (GA) matched normal placentae. Reference Gowland, Freeman and Issa93,Reference Duncan, Gowland, Francis, Moore, Baker and Johnson94,Reference Sinding, Peters and Frokjaer96–Reference Ingram, Morris, Naish, Myers and Johnstone99 The decrease in these relaxation times is most likely due to changes in tissue oxygenation and morphology. These changes can be the result of the presence of problems like infarction or necrosis. Placental T1 has also been investigated in cases of maternal hyperoxia. In both normal and dysfunctional placentae, the hyperoxic T1 increased, representing an increase of PO2 in blood and tissue. Reference Ingram, Morris, Naish, Myers and Johnstone99–Reference Ingram, Hawkins and Morris101 Furthermore, fetal liver T2* relaxation times were found to increase following maternal oxygenation. Reference Semple, Wallis and Haggarty102 Both vascular and placental relaxometry are sensitive to fetal oxygen change and can be used to assess oxygenation in cases of DOHaD.
Limitations with vascular relaxometry concern the biophysical models used to determine blood hematocrit and oxygen saturation. These limitations include the magnetic susceptibility differences between adult and fetal hemoglobin, Reference Yadav, Buch and Krishnamurthy88 physiological variations between participants, Reference Portnoy, Osmond, Zhu, Seed, Sled and Macgowan85 and bias from methemoglobin, which can decrease relaxation times. Reference Portnoy, Seed, Sled and Macgowan86 Despite these limitations, vascular relaxometry can be useful in many clinical applications, and the next step can potentially be used to optimize the delivery timing of late-onset growth-restricted fetuses. Reference Zhu, Milligan and Keating87
Placental microstructure
In addition to oxygenation, the microstructure of the placenta can be assessed using relaxometry. A negative correlation exists between placental T1 and GA, placental T2 and GA, and placental T2* and GA in normal placentae. Reference Gowland, Freeman and Issa93–Reference Sinding, Peters and Frokjaer96 It has been speculated that the change in relaxation times is due to changes in morphology and function instead of changes in blood volume. Magnetization transfer ratio (MTR), which is the ratio of bound protons to total protons in tissue, is another quantitative measure used to investigate placental morphology. MTR reflects the non-vascular component of the placental volume relative to total placental volume. Ong et al. did not find placental MTR to vary significantly between GA groups or between normal pregnancies and those affected by FGR and pre-eclampsia. Reference Ong, Tyler and Moore103 These findings suggest that total placental volumes are maintained regardless of GA and the presence of FGR or pre-eclampsia. In short, both relaxometry and MTR can provide additional insight into placental composition, which could reflect placental adaptations to the in utero environment at a macromolecular level.
Diffusion, perfusion, and flow
It can be challenging to identify a growth-restricted fetus in utero. There are still many cases of FGR that are left undiagnosed because current clinical methods for identification, such as umbilical artery Doppler ultrasound, fail to identify these fetuses, especially in obese women. Reference Lauring, Gupta, Kunselman, Repke and Pauli104 The ability to reliably and consistently identify FGR fetuses is critical for monitoring and early intervention. Reference Javor, Nasel, Schweim, Dekan, Chalubinski and Prayer105 Since the placenta provides nutrients and oxygen to the developing fetus via the maternal blood supply, any reduction in the placenta’s blood flow and perfusion may result in FGR. Reference Ludwig, Fain and Nguyen106,Reference Chou, Yeh and Chen107 To improve clinical diagnostic capabilities for FGR and better understand placental function, several studies have used specialized MRI techniques to investigate normal and abnormal perfusion across placental compartments and assess microstructure and microvasculature. These techniques include diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), intravoxel incoherent motion (IVIM), arterial spin-labeling (ASL), and phase-contrast MRI (PC-MRI).
DWI and DTI are diffusion-based techniques that characterize water molecules’ movement within tissues to investigate placental microstructure (see Fig. 2 for example images). DWI is sensitive to differences in the magnitude of water molecule diffusion (s/mm2), while DTI also accounts for the direction of water molecule diffusion. Reference Meoded, Poretti, Mori and Zhang108 Slator et al. assessed diffusion in the healthy placenta and found differences across placental compartments, indicating distinct microvascular and tissue microstructure in the maternal and fetal sides of the placenta. Reference Slator, Hutter and McCabe109 Diffusion MRI has been used to quantify the putative functional placental tissue (PFPT) volume, which is determined by high diffusion-weighted signal intensity in the placenta. Reference Javor, Nasel, Schweim, Dekan, Chalubinski and Prayer105 Diffusion parameters (diffusivity and fractional anisotropy) are reduced in the PFPT of placental FGR compared to healthy controls, suggesting the development of the villous network is affected in placental FGR. Reference Javor, Nasel, Schweim, Dekan, Chalubinski and Prayer105 The volume of PFPT decreases significantly in placental FGR compared to healthy controls, while brain volume measured from diffusion images remains similar, indicating a brain sparing effect. Reference Javor, Nasel, Dekan, Gruber, Chalubinski and Prayer110 DWI and DTI may provide insight into placental villous development changes in examples of negative fetal programming, such as FGR.

Fig. 2. Example of diffusion-weighted images of third-trimester pregnancy with different diffusion weightings [(A) b = 0 s/mm2, (B) b = 35 s/mm2, and (C) b = 750 s/mm2]. It is possible to estimate diffusion and perfusion in tissues by performing a bi-exponential fit of MRI data with different diffusion weightings. Figure courtesy of C. Rockel and the Pregnancy Research Group.
While IVIM is a type of DWI, it differs in technique as IVIM utilizes additional parameters (perfusion fraction, pseudo-diffusion related to blood microcirculation) to characterize water molecule movement within each image voxel to provide a measure of perfusion and diffusion. Reference Siauve, Chalouhi and Deloison111 IVIM has poor sensitivity in low blood volume areas but performs well in high blood volume regions such as the placenta. It was shown to be a reproducible technique for measuring placental and fetal lung and liver perfusion, perfusion fraction, and diffusion. Reference Jakab, Tuura, Kottke, Kellenberger and Scheer112 Perfusion fraction measurements were less reliable, particularly in the kidneys and brain, due to small organ size and fetal motion. Reference Jakab, Tuura, Kottke, Kellenberger and Scheer112 It should be noted that the choice of MRI field strength (1.5 vs. 3.0 Tesla) affected the measured perfusion fraction of liver and lung, with higher perfusion fractions being measured at 3.0 Telsa. Reference Jakab, Tuura, Kottke, Kellenberger and Scheer112 These results highlight that IVIM is a difficult imaging technique to perform, particularly in the moving fetus’s small organs. Despite these limitations, IVIM has been used to measure decreased perfusion fraction across the placenta in small for gestational age (SGA) compared to healthy controls in the second trimester. Reference Derwig, Lythgoe and Barker113 IVIM measures of perfusion in healthy placentae showed a higher perfusion fraction in the outer (maternal) zone than the inner (fetal) zone. Reference Moore, Strachan and Tyler114 The difference in perfusion is reduced in FGR compared to control pregnancies, as there is a reduction of placental perfusion fraction in the outer zone and slightly elevated perfusion fraction in the inner zone. Reference Moore, Strachan and Tyler114 It should be noted that these studies were performed at single sites with relatively small numbers of patients, so larger multicenter studies are needed to confirm the utility of IVIM for fetoplacental assessment.
ASL magnetically labels the water molecules found in arterial blood to create an endogenous contrast agent to quantify perfusion (ml/min/g). Reference Siauve, Chalouhi and Deloison111 ASL has been used to investigate placental perfusion in healthy placentae of animals Reference Ludwig, Fain and Nguyen106 and humans. Reference Derwig, Lythgoe and Barker113,Reference Shao, Liu and Martin115–Reference Gowland, Francis and Duncan117 It has shown spatial heterogeneity in perfusion, which may be related to the cotyledons of the placenta. Reference Ludwig, Fain and Nguyen106,Reference Shao, Liu and Martin115–Reference Gowland, Francis and Duncan117 Hutter et al. used ASL in combination with T2* measurements to quantify placental perfusion and oxygenation in the healthy placenta. Reference Hutter, Harteveld and Jackson116 ASL has also been used to identify SGA fetuses in the second trimester by measuring lower placental perfusion in SGA fetuses compared to healthy controls. Reference Derwig, Lythgoe and Barker113 Assessment of perfusion through IVIM or ASL in the placenta provides researchers and clinicians additional methods to probe placental function and adaptations.
PC-MRI is a technique used to measure flow on a larger scale than the perfusion measured by IVIM or ASL. PC-MRI is used to measure blood flow velocity (cm/s) as a function of time in either individual slices Reference Prsa, Sun and van Amerom118 or over a 3D volume. Reference Markl, Frydrychowicz, Kozerke, Hope and Wieben119 From these data, it is possible to quantify the time-varying blood flow (ml/s) through vessels. PC-MRI has been used to determine ranges of blood flow through major fetal vessels in healthy pregnancies. Reference Prsa, Sun and van Amerom118 3D PC-MRI (also known as 4D Flow) has been performed in pregnant large animal models to measure in- and out-flow of the uterus, umbilical cord, and fetal heart Reference Macdonald, Corrado and Nguyen120 and measure flow through major fetal vessels including the ductus venosus and ductus arteriosus. Reference Schrauben, Saini and Darby121 Flow through the uterine arteries was also measured in human pregnancies, with velocity measurements agreeing with those obtained with Doppler US. Reference Hwuang, Vidorreta and Schwartz122 A strength of PC-MRI is the ability to assess blood flow in a 3D volume, which could allow assessment of placental and cardiovascular adaptations throughout gestation as a result of fetal programming.
A common limitation of diffusion and perfusion MRI is the lack of consensus in the choice of models used to fit placental diffusion data; however, Slator et al. have proposed an anisotropic IVIM model that explains human placental diffusion data better than the apparent diffusion coefficient, IVIM and DTI models. Reference Slator, Hutter and McCabe109 There are a lack of data validating the diffusion and perfusion results against accepted clinical techniques, and some diffusion analysis methods can be adversely affected by placental location within the uterus. Reference Javor, Nasel, Schweim, Dekan, Chalubinski and Prayer105,Reference Slator, Hutter and McCabe109 The blood vessels that can be assessed with PC-MRI are limited by spatial resolution, making it difficult to perform in small animal models. In the future, studies may focus on developing techniques for clinical use, assessing placental microstructure in normal and abnormal placentae, and assessing of placental perfusion dynamics. Such developments would help understand placental adaptations to different DOHaD-related conditions such as maternal obesity, diabetes, and altered fetal growth.
Metabolic MRI
Optimal fetoplacental metabolism is essential for the fetus’s development in utero, and metabolic dysfunction is a possible cause of FGR. Reference Sharma, Shastri and Sharma123 In this section, we focus on the metabolic implications of FGR, which are relevant to DOHaD as growth restriction is known to impact health status later in life. A large focus of metabolic imaging is brain development; therefore, many of the spectroscopy studies mentioned here investigate the developing brain’s metabolic conditions in utero. MR techniques can provide direct measurements of metabolism in vivo and may be used to identify biomarkers useful to detect differences between normal and abnormal fetoplacental metabolism quantitatively. Reference Arthurs and Gallagher124
Magnetic resonance spectroscopy (MRS) provides data on metabolites in vivo, allowing for noninvasive measurements related to fetal or placental tissue’s metabolic status. MRS measures the signal from hydrogen (1H) nuclei or other MR-sensitive nuclei, such as 31-phosphorus (31P). MRS is used to distinguish distinct molecules in a volume of interest based on their resonant frequencies and can be used to quantify the concentration of a metabolite by measuring the area under its spectral peak. Reference Arthurs and Gallagher124 Successful fetoplacental MRS experiments have been demonstrated using clinical MRI scanners at field strengths of 1.5 and 3.0 Tesla.
The first study to demonstrate 1H-MRS of the human placenta in vivo was published in 2012 by Denison et al., specifically looking at choline, which is associated with a normal cell turnover rate in the developing fetal brain. Reference Denison, Semple, Stock, Walker, Marshall and Norman125 The study found a 60-fold reduction in the choline/lipid ratio in FGR fetuses’ placentas, indicating placental failure and possible fetal hypoxia. Reference Denison, Semple, Stock, Walker, Marshall and Norman125 Glutamate and glutamine (Glx) are related to the production of nucleotides and amino sugars needed for cell proliferation and have also been studied via MRS in the human placenta. Macnaught et al. reported that FGR placentae were found to have a significantly lower Glx/choline ratio than healthy controls at the same GA, Reference Macnaught, Gray and Walker126 demonstrating the prospect of Glx as a biomarker of placental function. 31P-MRS has more recently been employed to study placental metabolism, with studies focusing on phosphodiester (PDE) and phosphomonoester (PME) metabolites important for cell membrane degradation and formation, respectively. This technique has been used to demonstrate an elevated PDE/PME ratio in the placenta for pregnancies with early-onset pre-eclampsia compared to healthy pregnancies, Reference Sohlberg, Wikstrom and Olovsson127 as well as a case of abnormally high PDE signal from the placenta of a recently deceased fetus. Reference Weindling, Griffiths, Garden, Martin and Edwards128
1H-MRS has more commonly been used to study fetal brain metabolism, specifically targeting metabolites such as N-acetylaspartate (NAA), which is involved in neuronal metabolism, and lactate, which is related to hypoxia as a possible result of placental insufficiency. Reference Charles-Edwards, Jan, To, Maxwell, Keevil and Robinson129 Various 1H-MRS fetal brain studies have reported differences between healthy and FGR fetuses, including elevated lactate found in brains of FGR fetuses Reference Sohlberg, Wikstrom and Olovsson127,Reference Charles-Edwards, Jan, To, Maxwell, Keevil and Robinson129,Reference Cetin, Barberis and Brusati130 and reduced NAA/choline and NAA/creatine ratios in FGR fetuses. Reference Azpurua, Alvarado, Mayobre, Salom, Copel and Guevara-Zuloaga131–Reference Story, Damodaram and Allsop133 Sanz-Cortes et al. included SGA fetuses in the study. They found that some SGA fetuses demonstrated reduced NAA/choline ratios similar to the FGR fetuses, Reference Sanz-Cortes, Simoes, Bargallo, Masoller, Figueras and Gratacos132 indicating that MRS may introduce a method of differentiating between metabolically healthy small fetuses and growth-restricted fetuses.
When studying complex systems like the fetus and placenta, it is useful to have spatial information to discern different metabolites’ locations. Although non-proton MRI is useful for measuring different metabolites, it is challenging to perform due to low endogenous signal compared to 1H. With the advancement of hyperpolarized (HP) MRI, it is possible to image non-proton molecules in a reasonable time frame by boosting the nuclear magnetic signal up to 10,000-fold. Reference Ardenkjaer-Larsen, Fridlund and Gram134
In vivo hyperpolarized MRI of the fetus or placenta is a very new field, and as such, there are only three animal studies in the literature to discuss. Friesen-Waldner et al. validated the technique of imaging injected hyperpolarized [1-13C]pyruvate and its downstream metabolites (lactate, alanine, and bicarbonate) in utero using 3D chemical shift imaging on a pregnant guinea pig model (Fig. 3). This study reported pyruvate and lactate signal from all 30 healthy placentas and fetal livers. Reference Friesen-Waldner, Sinclair and Wade135 A proof-of-concept paper by Markovic et al. focused on the use of the chinchilla as a model animal for fetoplacental research and used HP 13C MRI to observe lactate and pyruvate in the placentae of four healthy pregnancies. Reference Markovic, Fages and Roussel136 Lastly, Wang et al. investigated placental metabolism via HP 13C MRI in a Wistar rat model of pre-eclampsia and observed differences in urea kinetics and pyruvate to lactate metabolism in the placentae of pre-eclamptic pregnancies compared to healthy pregnancies. Reference Wang, Ohliger and Larson137 This study imaged three injected hyperpolarized substrates – [1-13C]pyruvate, 13C-bicarbonate, and 13C-urea – and reported observing urea, bicarbonate, pyruvate, lactate, and alanine in placentae, and urea in some fetal livers. It is important to note that HP 13C MRI has been performed successfully in non-pregnant human subjects in vivo. Reference Wang, Ohliger and Larson137 The validation of this technique in human patients combined with the success of fetoplacental imaging in multiple animal models suggests the obvious next step in this field is the translation to HP 13C MRI studies of human pregnancy.
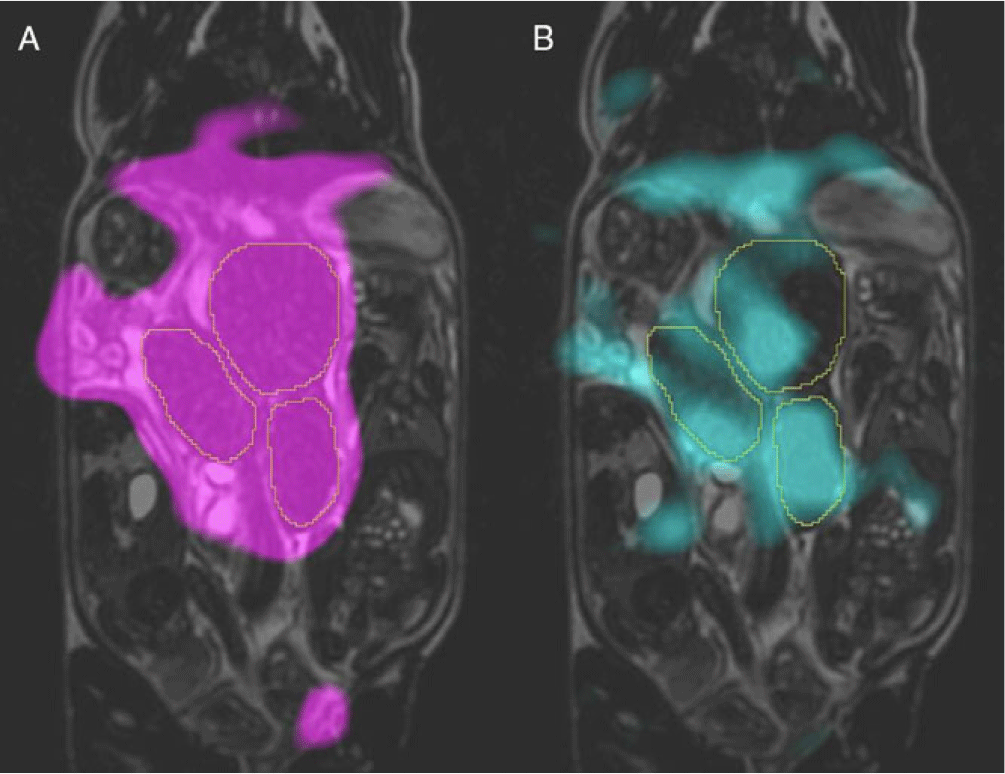
Fig. 3. Typical hyperpolarized 13C metabolite images overlaid on coronal T2 of the same guinea pig at 22.5 s post-injection of [1-13C]pyruvate solution. Images of signal from two metabolites are shown here: pyruvate is shown in magenta (A) and lactate in cyan (B). The placentae are outlined in each image Image courtesy of L. Smith, L. Friesen-Waldner, and T. Regnault.
Both MRS and hyperpolarized MRI have challenges associated with them. An obvious challenge is fetoplacental motion, which may lead to MRS signal detection outside of intended voxels and artifacts in HP MRI images. Another challenge is the need for specific hardware, such as specialized RF coils for non-proton MRS and MRI. Limitations specific to MRS include long scan times that are not clinically translatable, difficulty performing small animal studies due to the low sensitivity resulting from the small fetus/placenta size, and limited spatial coverage. HP 13C MRI limitations include the need for specialized equipment to hyperpolarize samples, limited resolution, and rapid signal decay that limits the temporal window for imaging post-injection.
Conclusions
While we understand that the environment a fetus experiences in utero can alter its later-life health through developmental programming, the mechanisms supporting the DOHaD hypothesis remain unclear. Imaging may help answer some of the questions regarding when and how this programming takes place. MRI is ideal for this task thanks to its ability to image different structures and function noninvasively during pregnancy.
Examining fetal growth and organ development allows for identifying under- or over-growth and deviations from normal organ development that may result from an adverse in utero environment. The ability to assess structural changes in adipose tissue and body composition can help determine if and when in gestation the programming is taking place, through alterations in lipid deposition resulting from altered nutrient availability. Measurement of placental and fetal oxygenation using MRI can provide insight into the placental tissue function and any fetal adaptations that compensate for deficiencies. Furthermore, MRI methods that assess diffusion and perfusion can provide additional insight into placental tissue structure and function. Finally, the ability to probe specific metabolites will help us understand the normal metabolic function of the fetus and placenta in utero. The information from the metabolites will also provide information about how the fetus and placenta can be altered in disease on a chemical level, making it one of the most powerful tools MRI has to offer the field of DOHaD.
This review of MRI used to study DOHaD has revealed a focus on FGR. However, we believe that MRI can help investigate other DOHaD-related metabolic or cardiovascular conditions, such as macrosomia, pre-eclampsia, maternal diabetes, and maternal obesity. It could also be a means of monitoring interventions aimed at reducing undesirable fetal programming throughout gestation.
Overall, MRI is a useful and diverse research tool for investigating the cardiovascular and metabolic consequences of DOHaD thanks to its excellent soft-tissue contrast and ability to quantify function in the fetus and placenta. The information it provides will help bridge the gap from the cellular mechanisms of programming to the clinical prevention of later-life disease.
Acknowledgments
The authors gratefully acknowledge support from Western University and Children’s Health Research Institute/Children’s Health Foundation.
Financial support
This work was supported by the Natural Sciences and Engineering Research Council of Canada (grant number RGPIN-2019-05708); the Canadian Institutes of Health Research (grant number 162308); and the National Institutes of Health (grant number 1U01HD087181-01).
Conflicts of interest
None.









