Introduction
The genus Polydrusus Germar (Coleoptera: Curculionidae) is composed of 188 species of herbivorous weevils that are rhizophagous (i.e., root-feeding) as larvae, with most being native to the Palaearctic region (Pierce Reference Pierce1916; Sleeper Reference Sleeper1957; Alonso-Zarazaga et al. Reference Alonso-Zarazaga, Barrios, Borovec, Bouchard, Caldara and Colonnelli2017). Four Polydrusus species are native to various parts of the United States of America and Canada and are known to feed only on a limited number of host plants. These weevil species include Polydrusus americanus (Gyllenhal) (senior synonym of Cyphomimus dorsalis Horn), Polydrusus decoratus Woodruff, Polydrusus hassayampus Sleeper, and Polydrusus ochreus (Fall) (Fig. 1A). Three additional polyphagous Polydrusus species were introduced to North America from Europe (Pierce Reference Pierce1916; Sleeper Reference Sleeper1957; Alonso-Zarazaga et al. Reference Alonso-Zarazaga, Barrios, Borovec, Bouchard, Caldara and Colonnelli2017). These include Polydrusus cervinus (Linnaeus), first recorded in 1963 in the United States of America and in 1987 in Canada (Warner Reference Warner1971; Bright Reference Bright1988); Polydrusus formosus (Mayer) (senior synonym of Polydrusus sericeus (Schaller)), first recorded in North America in 1916 (Pierce Reference Pierce1916); and Polydrusus impressifrons (Gyllenhal), first recorded in North America in 1906 (Parrott and Glasgow Reference Parrott and Glasgow1916; Frost Reference Frost1946). All three of these nonnative species have since spread throughout much of northeastern North America (Bright Reference Bright1988; Mattson et al. Reference Mattson, Niemela, Millers and Inguanzo1994; Arnett Reference Arnett2000; Majka et al. Reference Majka, Anderson, McAlpine and Webster2007a, Reference Majka, Anderson and McCorquodale2007b; Coyle et al. Reference Coyle, Mattson, Raffa, Johnson and Murray2008; Webster et al. Reference Webster, Anderson, Sweeney and DeMerchant2012, Reference Webster, Anderson, Webster, Alderson, Hughes and Sweeney2016; Larson Reference Larson2018; Fig. 1B, C, D).
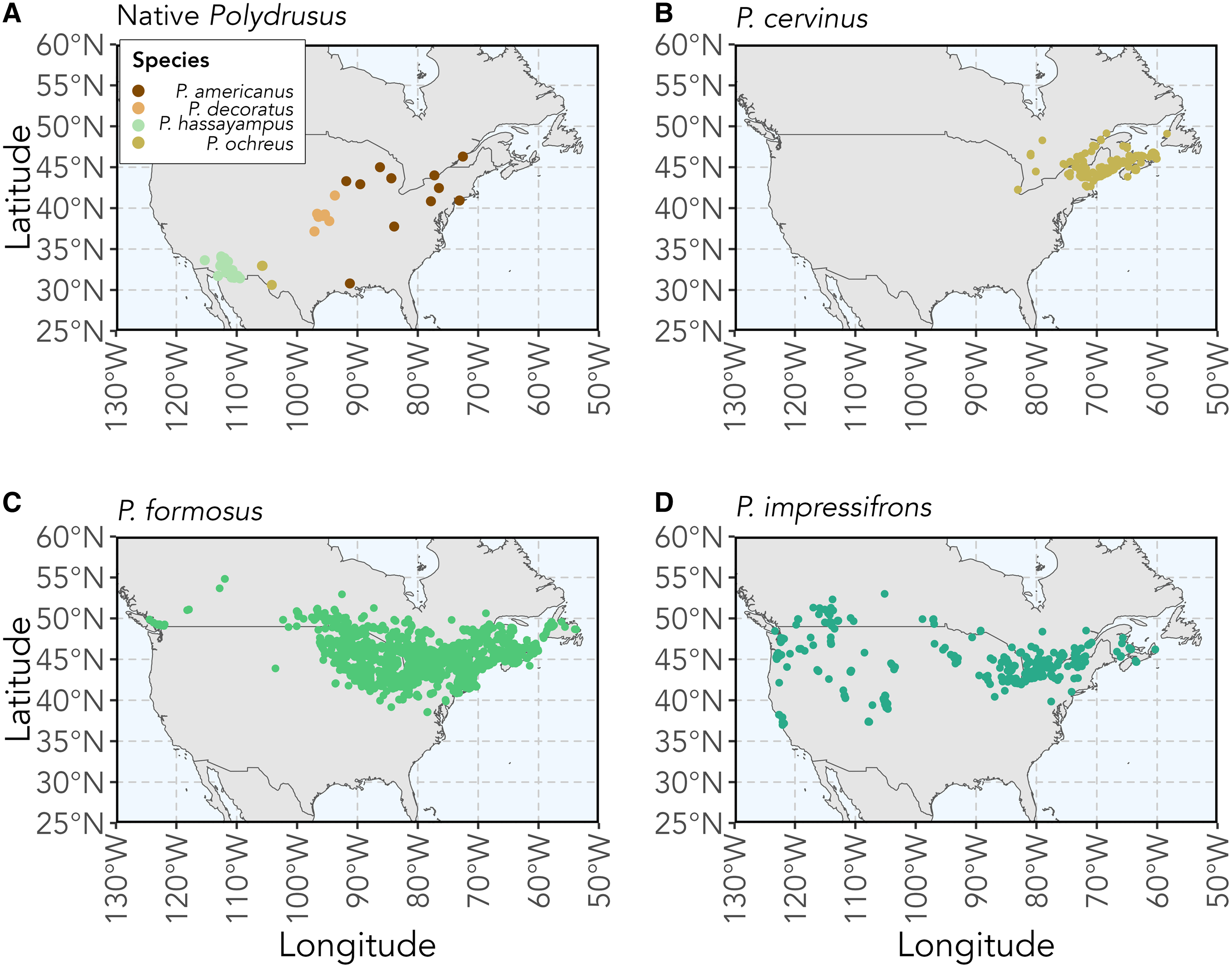
Figure 1. Maps of the recorded observations and collected specimen localities for A, the four Polydrusus species native to the United States of America and Canada, and for the three nonnative Polydrusus species to North America: B, P. cervinus; C, P. formosus; and D, P. impressifrons. Coordinate data for species were downloaded from Symbiota Collections of Arthropods Network (SCAN; https://scan-bugs.org/) and the Global Biodiversity Information Facility (GBIF; www.gbif.org). Methods for map creation and a complete list of sources, including data downloaded from the SCAN and GBIF used for the creation of the maps, can be found in Supplementary material, File S1.
The ecology and impacts of introduced weevils, such as the three nonnative Polydrusus species now found in North America, are understudied compared to other invasive insects. Previous studies on the ecology of Polydrusus species in North America span different contexts, such as the larvae of P. formosus as root-feeding pests within midwestern United States hardwood forests and adult P. impressifrons as foliar-feeding pests of hybrid poplar (Salicaceae) plantations (Populus trichocarpa Torrance and A. Gray ex. Hooker, Populus nigra Linnaeus, and Populus deltoides W. Bartram ex. Marshall P. nigra) in the Pacific Northwest (Arnett et al. Reference Arnett, Ross, Thomas, Skelley and Frank2002; Nordman et al. Reference Nordman, Robison, Abrahamson and Volk2005; Pinski et al. Reference Pinski, Mattson and Raffa2005a, Reference Pinski, Mattson and Raffa2005b; Majka et al. Reference Majka, Anderson, McAlpine and Webster2007a, Reference Majka, Anderson and McCorquodale2007b; Coyle et al. Reference Coyle, Mattson, Raffa, Johnson and Murray2008, Reference Coyle, Mattson and Raffa2010, Reference Coyle, Allred, Kosola and Raffa2011, Reference Coyle, Mattson, Jordan and Raffa2012; Hillstrom et al. Reference Hillstrom, Vigue, Coyle, Raffa and Lindroth2010; Humble and Hueppelsheuser Reference Humble and Hueppelsheuser2012; Webster et al. Reference Webster, Anderson, Sweeney and DeMerchant2012; Niedbala Reference Niedbala2013; Rodstrom Reference Rodstrom2013; Niedbala et al. Reference Niedbala, Rodstrom and Brown2017). Adults of both species feed on buds and foliage and can cause considerable damage to tree crops (Pinski et al. Reference Pinski, Mattson and Raffa2005b; Niedbala Reference Niedbala2013; Niedbala et al. Reference Niedbala, Rodstrom and Brown2017). Adults of P. impressifrons can damage buds and leaves in production plantings and nurseries of young hybrid poplar in areas of eastern Oregon and Washington, United States of America, and can rapidly recolonise stands after insecticide treatment (Nordman et al. Reference Nordman, Robison, Abrahamson and Volk2005; Webster et al. Reference Webster, Anderson, Sweeney and DeMerchant2012; Niedbala Reference Niedbala2013; Rodstrom Reference Rodstrom2013; Niedbala et al. Reference Niedbala, Rodstrom and Brown2017). On a tree farm in Oregon in 2010, for example, 30% of all newly planted cuttings of hybrid poplar exhibited stunted growth or died from P. impressifrons damage (Rodstrom Reference Rodstrom2013). Studies in Wisconsin, United States of America, estimate that introduced rhizophagous weevils, including P. formosus, consume an estimated 15% of the fine-root masses of forest plants, and the authors hypothesised that the insects may be displacing native weevil fauna due to their numerical abundance (Pinski et al. Reference Pinski, Mattson and Raffa2005a; Coyle et al. Reference Coyle, Mattson, Raffa, Johnson and Murray2008).
Hybrid hazelnuts, Corylus americana Walter × Corylus avellana Linnaeus (Betulaceae), are a novel crop and fast-spreading industry for the north-central region of North America (Braun et al. Reference Braun, Demchik, Fischbach, Turnquist and Kern2019), with approximately 400 growers across the region as of 2023 (Lois Braun, personal communication). Polydrusus formosus larvae and adults both use hazels, Corylus spp. Linnaeus, as host plants: larvae are considered serious root-feeding pests in young plantings of hazels in Belgium, and adults have been noted as abundant on hazel in the United Kingdom as well as in the United States of America on wild stands of C. americana in Indiana (Frost Reference Frost1946; Morris Reference Morris1978; Casteels and de Clercq Reference Casteels and de Clercq1988). In addition, in laboratory feeding trials, adult P. formosus preferred feeding on the foliage of trees in the birch family (Betulaceae, same family as Corylus) over other hardwood trees (Pinski et al. Reference Pinski, Mattson and Raffa2005b). Because both Polydrusus species can damage foliage and buds of fruit trees in Europe, there is concern that these species could emerge as pests to the developing hazelnut industry in the midwestern United States (Parrott and Glasglow Reference Parrott and Glasgow1916; Pierce Reference Pierce1916). Both nonnative P. formosus and P. impressifrons were abundant in five hybrid hazelnut orchards across Minnesota and Wisconsin, United States of America, between 2017 and 2021 (Chediack et al. Reference Chediack, Liesch, Shanovich and Aukema2022), for example. Understanding the ecology of potential pests is key to the success of hybrid hazelnut production (AliNiazee Reference AliNiazee1997, Reference AliNiazee1998; Chediack et al. Reference Chediack, Liesch, Shanovich and Aukema2022; Perish et al. Reference Perish, Shanovich, Koch, Lindsey and Aukema2023).
All nonnative Polydrusus species exhibit similar life cycles and host plant breadths (Parrott and Glasgow Reference Parrott and Glasgow1916; Pierce Reference Pierce1916; Pinski et al. Reference Pinski, Mattson and Raffa2005a, Reference Pinski, Mattson and Raffa2005b; Coyle et al. Reference Coyle, Mattson, Raffa, Johnson and Murray2008). Adults are diurnal and emerge in the late spring and early summer, when they immediately begin preovipositional feeding on the developing buds and leaves of various deciduous woody plants (Parrott and Glasgow Reference Parrott and Glasgow1916; Pierce Reference Pierce1916). Mating and oviposition occur approximately two weeks after emergence (Parrott and Glasgow Reference Parrott and Glasgow1916; Pierce Reference Pierce1916; Coyle et al. Reference Coyle, Mattson, Raffa, Johnson and Murray2008; Niedbala Reference Niedbala2013). Adult females oviposit small white cylindrical eggs, about 0.53 × 0.32 mm in size, either singly or in clutches of up to 85 eggs, into grooves of the bark of host trees or the surface of the soil (Parrott and Glasgow Reference Parrott and Glasgow1916; Pinski et al. Reference Pinski, Mattson and Raffa2005b; Coyle et al. Reference Coyle, Mattson, Raffa, Johnson and Murray2008). In laboratory trials, P. formosus produced approximately 830 eggs over a period of 25.8 days when feeding on optimal host plants (Pinksi et al. Reference Pinski, Mattson and Raffa2005b). Eggs hatch about one month later, after which the larvae burrow into the soil to a depth of 10 cm, where they actively feed on tree roots until the following spring, when they pupate (Pinski et al. Reference Pinski, Mattson and Raffa2005b; Coyle et al. Reference Coyle, Mattson, Raffa, Johnson and Murray2008; Niedbala Reference Niedbala2013). However, despite shared ecologies and the presence of multiple Polydrusus species in the United States of America, little is known about how they might co-occur or impact the same host plant.
Here, we present compiled distribution and host plant information of the Polydrusus species of the United States of America and Canada. Moreover, we provide a compendium of species descriptions for distinguishing between and among native and nonnative species and sexes of nonnative Polydrusus species. We also report the seasonal phenology and sex ratios of adult P. formosus and P. impressifrons weevils within two hybrid hazelnut orchards in Minnesota in 2020 and 2021. Sex ratios influence population growth rates and, therefore, are crucial information for development of pest management guidelines (Gou et al. Reference Gou, Wang, Quandahor, Liu and Liu2019). These findings will aid in the development of integrated pest management plans should they become necessary for nonnative Polydrusus species in agricultural and natural resource settings.
Methods
Polydrusus species descriptions and distributions
Coordinate data for all seven Polydrusus species were downloaded from the Symbiota Collections of Arthropods Network (SCAN; https://scan-bugs.org/; download 2022) and the Global Biodiversity Information Facility (GBIF; www.gbif.org; download 2022). Methods for map creation and a complete list of sources, including the complete datasets downloaded from SCAN and GBIF, can be found in Supplementary material, File S1. Species descriptions and global records of host plants for the seven Polydrusus spp. were compiled via a thorough search of historical and contemporary literature on Google Scholar and Web of Science, using keywords such as “Polydrusus” and “North America.”
Seasonal phenology of nonnative Polydrusus weevils in hybrid hazelnut orchards
Research sites
This study was conducted over a two-year period at two experimental hazelnut orchards in Minnesota: one located in Rosemount (44° 43′ 36″ N, 93° 05′ 59″ W) and one in Saint Paul (44° 59′ 53″ N, 93° 10′ 30″ W). The planting in Rosemount is 0.18 ha and is composed of two agronomic trials: one, planted in 2011, consisting of 180 hybrid hazel plants representing 12 genotypes spaced 3 m between rows and 1.5 m between plants, and the other trial, planted in 2013, consisting of 90 plants representing six hybrid hazel genotypes spaced approximately 3 m between rows and 2 m between plants. The planting in Saint Paul was planted in 2008, is 0.68 ha, and is composed of a germplasm trial of top hybrid hazel selections from farms throughout the Midwest and Canada, as well as a few pure C. americana and C. avellana genotypes, with a total of 149 genotypes represented in the planting in triplicate. No insecticides have been applied to the research sites since 2012, and only annual spot treatments of glyphosate were used for weed control. Daily temperatures were recorded at each planting via HOBOware temperature data loggers (Onset Computer Corporation, Bourne, Massachusetts, United States of America) that were deployed for the course of the entire field season each year. Accumulated growing degree days (GDD), a measurement of heat accumulation, were calculated starting 1 January of each year. We used a lower development threshold of 8.8 °C, the minimum threshold for strawberry root weevil, Otiorhynchus ovatus (Linnaeus) (Coleoptera: Curculionidae: Entiminae). Otiorhynchus ovatus is the closest related species (same subfamily as Polydrusus) with an established lower development threshold (Blodgett and Grey Reference Blodgett and Grey2016).
Sampling of Polydrusus weevils
To quantify Polydrusus phenology and abundance in the crop, we sampled two hybrid hazel plants of six different genotypes at each location weekly (i.e., 12 plants at each location; 24 plants total). Sampling occurred between 27 May and 23 July 2020 and 25 May and 20 July 2021, which encompassed the abundance trends based on previous observations. Adult weevils were collected via beat-sheet sampling, an effective method for collecting Polydrusus (Pinski et al. Reference Pinski, Mattson and Raffa2005a). Both in-row sides of each plant were sampled by inserting a wooden dowel (1 m long × 2.5 cm diameter) into the plant and beating both halves of the plant 10 times onto a 1-m2 canvas. Two people were needed for this process: one beat the plant and the other held the canvas below the branches. Polydrusus that fell onto the canvas were then placed in resealable plastic bags, labelled by date and tree identifier, and stored at 18 °C until further processing. This dataset has been deposited in the Data Repository of the University of Minnesota (St. Paul, Minnesota; Shanovich et al. Reference Shanovich, Lisak, Lindsey and Aukema2023).
Identification of specimens
Collected Polydrusus specimens were identified to species and sexed morphologically by authors Lisak and Shanovich with guidance from Sleeper (Reference Sleeper1957) and Arnett et al. (Reference Arnett, Ross, Thomas, Skelley and Frank2002) and by comparison to voucher specimens of P. formosus (determined by Steven Katovich and Gregory P. Setliff) and P. impressifrons (determined by Roger J. Blahnik and Ralph W. Holzenthal) from the University of Minnesota Insect Collection. A stereo microscope at 30× magnification was used to assist in identification. Ten random specimens from both of the two identified species (i.e., 20 specimens in total) were sent to Patrick Leisch, director of the insect diagnostics laboratory at the University of Wisconsin – Madison, for confirmation of the authors’ determinations. Leisch used the taxonomic key from Bright and Bouchard (Reference Bright and Bouchard2008) for his confirmations with guidance from Sleeper (Reference Sleeper1957) and voucher specimens from his personal collection. The specimens were deposited into the Wisconsin Insect Research Collection (specimen numbers from WIRC00193058 through WIRC00193077; University of Wisconsin – Madison, Madison, Wisconsin).
In addition, molecular species identification (i.e., DNA barcoding) was used on representative specimens of each species to confirm morphological identities. The DNA was extracted from adult specimens of P. impressifrons and P. formosus collected on 2 July 2020 using nondestructive HotSHOT DNA extraction (adapted from Truett et al. Reference Truett, Heeger, Mynatt, Truett, Walker and Warman2000; New England BioLabs Inc., Ipswich, Massachusetts, United States of America). The barcoding fragment of cytochrome C oxidase subunit 1 (CO1) was amplified by polymerase chain reaction with 500 nM each of LCO and HCO primers (Folmer et al. Reference Folmer, Black, Hoeh, Lutz and Vrijenhoeck1994) and 1 μL of DNA template in 20-μL reactions with Q5 Hot Start High-Fidelity 2× master mix (New England BioLabs Inc.) following the manufacturer’s protocol. Polymerase chain reaction products were run on a 1% agarose gel, stained postelectrophoresis with GelRed (Biotium, Fremont, California), cleaned for sequencing with DNA Clean and Concentrator-5 columns (Zymo Research Company, Orange, California), and Sanger-sequenced in both directions. Sequence reads were manually inspected for peak quality, contigs were aligned, and primer sequences trimmed in SnapGene (https://www.snapgene.com/). Consensus sequences were used to query the GenBank nonredundant database with nucleotide blast (blastn) using default parameters (https://blast.ncbi.nlm.nih.gov/). Finally, we checked our barcodes against the Barcode of Life Data Systems database (https://www.boldsystems.org/) to determine the BIN assignment of each barcode sequence.
Statistical analysis
All analyses were conducted with R, version 4.1.1 (R Core Team 2021), and RStudio, version 4.1.0 (RStudio Team 2021). We report the abundance of individuals for each of the two Polydrusus species by year. For the response variable, samples from both in-row sides of each plant per date were summed. To avoid zero-inflation and overdispersion in the data, we omitted from the analyses five plants across the two sites (four plants from Rosemount and one from Saint Paul) in which we had collected no Polydrusus individuals on more than 17 sampling events out of the total 20 sampling events across years. To assess whether the abundance of total numbers of Polydrusus weevils differed between years, we used a generalised linear mixed effect model with a Poisson response, terms for species and year as fixed effects, and term for plant nested within site as a random effect to contend with potential temporal autocorrelation from resampling of the hybrid hazel plants throughout each year (package, code, citation: lme4, glmer; Bates et al. Reference Bates, Mächler, Bolker and Walker2015). Numbers of Polydrusus were found to differ between years because of the underlying differences in distributions (reported in results below). Years were therefore analysed separately, and site was removed from the random effect term as it contributed to no significant variation within the model error. To assess whether abundance between Polydrusus species differed within each year, we used generalised linear mixed effect models, with a term for species as a fixed effect. A term for hybrid hazel plant nested within site was also included as a random effect in 2020. In 2021, site contributed no significant variation to the models; therefore, only a term for plant was included (package, code, citation: lme4, glmer; Bates et al. Reference Bates, Mächler, Bolker and Walker2015). Then, to assess whether sex ratios within a species differed each year, we performed logistic regressions using generalised linear models, with a binomial response and term for species as a fixed effect and a term for plant nested within site as random effects (package, code, citation: stats, glm; R Core Team 2021). Random effects of site were removed from the models because they did not contribute significant variation. Mean separation was performed for statistically significant fixed effects by calculating estimated marginal means and R 2 values when appropriate (packages, code, citations: emmeans, emmeans; Lenth Reference Lenth2023; MuMIn, r.squaredGLMM; Bartoń Reference Bartoń2023).
Results
Species descriptions of adult Polydrusus species of the United States of America and Canada
Native species
Polydrusus americanus has a body length of 3.5–4.8 mm and body width of 1.5–2.0 mm (Sleeper Reference Sleeper1957; Fig. 2A). The body is covered in dark reddish-brown scales and speckled with the marking of white scales; colour pattern more sharply defined than in other species (Sleeper Reference Sleeper1957; Fig. 2A). The species is distributed from southeastern Canada to the southeastern United States of America and west to Louisiana and Minnesota (Fig. 1A). Although coordinate data could not be found for certain states and are therefore not shown on the map, this species has also been recorded in Kansas, Maryland, Michigan, Missouri, Ohio, Wisconsin, and West Virginia in the United States of America and in Ontario and Québec in Canada (O’Brien and Wibmer Reference O’Brien and Wibmer1982; Fig. 1A). The species has been found commonly on beech (Fagus spp. Linnaeus) and recorded on other tree species as well (Blatchley and Leng Reference Blatchley and Leng1916; Supplementary material, Table S1).

Figure 2. Lateral and dorsal views of native and nonnative Polydrusus weevils of the United States of America and Canada, all with 1 mm scale bars: A, P. americanus (photos by M.J. Hatfield); B, P. decoratus (photos by Sangmi Lee); C, P. hassayampus (photos by Glenn Seplak); D, P. ochreus; E, P. cervinus (photos by Nico Franz and Sangmi Lee); F, P. formosus (photos by Pheylan Anderson and Sarah C. Lisak, University of Minnesota); and G, P. impressifrons (photos by Pheylan Anderson and Sarah C. Lisak, University of Minnesota). Scale bars in each panel are 1 mm.
Polydrusus decoratus has a body length of 3.10–3.75 mm and body width of 1.25–1.40 mm (Sleeper Reference Sleeper1957; Fig. 2B). The body is brown to brownish black, with black abdominal segments, and is completely covered by greyish-white, ashy, and light brown scales (Woodruff Reference Woodruff1923; Fig. 2B). On the elytra, the light brown scales form a distinct three-lobed patch that is sometimes outlined with darker brown scales (Woodruff Reference Woodruff1923; Fig. 2B). The species is distributed throughout the central midwestern region of the United States of America and recorded in Alabama and Missouri, although these latter occurrences are not shown on the map due to a lack of coordinate data (Sleeper Reference Sleeper1957; O’Brien and Wibmer Reference O’Brien and Wibmer1982; Fig. 1A). Adults are found abundantly on sugar maple, Acer saccharum Marshall (Sapindaceae) but also are recorded on chestnut oak, Quercus prinus Linnaeus (Fagaceae) (Woodruff Reference Woodruff1923).
Polydrusus hassayampus has a body length of 2.4–3.6 mm and body width of 1.0–1.7 mm (Sleeper Reference Sleeper1957; Fig. 2C). The body, legs, and antennae are reddish brown but are mostly concealed by a dense coat of metallic green scales (Sleeper Reference Sleeper1957; Fig. 2C). The species is distributed across the southwestern United States of America (Fig. 1A). Adults have been found on palo verde, Parkinsonia aculeata Linnaeus (Fabaceae) in the southwestern United States (Arizona State University Biocollections 2023).
Polydrusus ochreus has a body length of 3–4 mm and body width of 1.4–1.6 mm (Sleeper Reference Sleeper1957; Fig. 2D). The body is reddish brown with orange legs and is covered in pale yellow scales (Fall and Cockerell Reference Fall and Cockerell1907; Fig. 2D). The species is distributed across the southwestern United States of America (O’Brien and Wibmer Reference O’Brien and Wibmer1982; Fig. 1A). In New Mexico, adults are commonly found on Gambel oak, Quercus gambelii Nuttall (Fagaceae) (Pierce Reference Pierce1916).
Nonnative species
Polydrusus cervinus has a body length of 4–5 mm (Warner Reference Warner1971; Fig. 2E). The body is black and covered in scales ranging in colour from copper to blue-green, and the elytra are mottled in bare black patches, forming a distinct “checkered” appearance (Warner Reference Warner1971; Fig. 2E). The species is distributed throughout northeastern United States of America and Canada and is recorded in Newfoundland and Labrador, although these latter occurrences are not shown on the map due to a lack of coordinate data (O’Brien and Wibmer Reference O’Brien and Wibmer1982; Webster et al. Reference Webster, Anderson, Webster, Alderson, Hughes and Sweeney2016; Langor et al. Reference Langor, Anderson, Bouchard and Langor2022; Fig. 1B). Adults are found on many species of broadleaved trees and shrubs (Supplementary material, Table S1).
Polydrusus formosus has a body length of 5.3–6.8 mm and width of 1.8–2.75 mm (Sleeper Reference Sleeper1957; Fig. 2F). The body is black and covered in opaque green scales (Pierce Reference Pierce1916; Fig. 2F). The species is distributed throughout southeastern Canada and the northeastern United States of American, with some additional records in Alberta, British Columbia, and Newfoundland and Labrador, although these latter occurrences are not shown on the map due to a lack of coordinate data (Langor et al. Reference Langor, Anderson, Bouchard and Langor2022; Fig. 1C). Adults are found on a wide variety of trees and shrubs (Supplementary material, Table S1).
Polydrusus impressifrons has a body length of 3.7–5.5 mm and width of 1.3–2.2 mm (Sleeper Reference Sleeper1957; Fig. 2G). Body is covered in distinctly green scales (Sleeper Reference Sleeper1957; Fig. 2G). The species is distributed throughout the southern part of Canada and northern half of the United States of America and in California and Colorado (Webster et al. Reference Webster, Anderson, Sweeney and DeMerchant2012; Larson Reference Larson2018; Fig. 1D). Adults have been found on many species of trees and shrubs but are thought to be especially common on aspen, Populus spp. Linnaeus, willow, Salix spp. Linnaeus (Salicaceae), and members of the birch family (Betulaceae) (Parrott and Glasgow Reference Parrott and Glasgow1916; Pierce Reference Pierce1916; Dieckmann Reference Dieckmann1980; Supplementary material, Table S1).
Distinguishing between Polydrusus formosus and Polydrusus impressifrons
Note that P. formosus and P. impressifrons are morphologically similar. For this reason, we provide here additional characteristics to distinguish them, with the goal of increasing accessibility for nontaxonomists (e.g., land managers, hazel growers).
Specimens of P. formosus can be larger than P. impressifrons (see descriptions for each above; Sleeper Reference Sleeper1957). The positioning of the eyes and scales on each species’ head differs (Sleeper Reference Sleeper1957). Polydrusus formosus have dorsal-positioned eyes, with a long seam-like crevice between the eyes and spaced-out, elongated scales that swirl away from the middle of the pronotum (Fig. 3A). In contrast, P. impressifrons have laterally positioned eyes, with a circular crevice between the eyes and a dense pattern of round scales on the pronotum (Fig. 3B). Lastly, P. impressifrons has a more flattened and broadened frons than P. formosus does, which exhibits more of a raised and narrow frons (Sleeper Reference Sleeper1957; Fig. 3A, B).

Figure 3. Photos for distinguishing between adult specimens of Polydrusus formosus and P. impressifrons and sex identification of each species: A, Polydrusus formosus eye and scale positioning; B, Polydrusus impressifrons eye and scale positioning; C, Polydrusus formosus distinct elytral striation and punctation patterns; D, Polydrusus impressifrons distinct elytral striation and punctation patterns, the apex of the last ventral sternite; E, in a female specimen of P. formosus; F, in a female specimen of P. impressifrons; G, in a male specimen of P. formosus; and H, in a male specimen of P. impressifrons (photos by Sarah C. Lisak).
Pierce (Reference Pierce1916) translated and compiled original species descriptions from Gyllenhal (Reference Gyllenhal, Schrönherr, Gyllenhal and Boheman1834) and von Fåhræus (Reference von Fåhræus1871) that describe the elytra of P. formosus as “moderately punctate-striate” (i.e., striated with dots) and P. impressifrons as “subtly punctate-striate.” Aside from the differences in severity of the punctations and striations on the two species’ elytra, the patterns of punctations and striations differ (Fig. 3C, D): the elytra of P. formosus have pronounced striation, with some striates appearing to be comprised of punctations, whereas the elytra of P. impressifrons have less pronounced striations and its punctations occur between the striates (Fig. 3C, D).
It should be noted that the length of the antennal scape is another distinguishing characteristic between the two species that is mentioned in Bright and Bouchard (Reference Bright and Bouchard2008); Patrick Liesch used this characteristic in his taxonomic confirmation of our identifications. The antennal scape of P. formosus extends to the posterior margin of the eye, whereas the antennal scape of P. impressifrons extends well beyond the posterior margin of the eye (Bright and Bouchard Reference Bright and Bouchard2008).
Sex identification of Polydrusus formosus and Polydrusus impressifrons
We identified characteristics for identifying the sex of Polydrusus spp. by focusing on the apex of the last ventral sternite (Fig. 3E, F, G, H). The last ventral sternite of females of both P. formosus and P. impressifrons is completely rounded and smooth (Fig. 3E, F). Additionally, the P. formosus female sternite is often characterised as translucent and brown in colour (Fig. 3E, F). The sternites of males of both species possess a concave notch. In P. formosus males, the notch is shallower, whereas P. impressifrons males have a V-shaped crevice with very few scales (Fig. 3G, H).
Seasonal phenology of nonnative Polydrusus weevils in hybrid hazelnut orchards
Over the two years, we collected a total of 1410 adult Polydrusus weevils during 323 sampling events. All of these adult weevils were identified as either P. formosus or P. impressifrons. A total of 1080 of the weevils (687 P. formosus and 393 P. impressifrons) were collected in 2020 (derived from 152 sampling events), and 330 weevils (193 P. formosus and 137 P. impressifrons) were collected in 2021 (derived from 171 sampling events). Adults of both species were initially collected the weeks of 30 May 2020 (245 GDD) and 29 May 2021 (281 GDD), and the last days of collection for adults of each species took place the weeks of 25 July 2020 (1005 GDD) and 24 July 2021 (1141 GDD; Fig. 4). In 2020, peak emergence of adult P. impressifrons occurred the week of 6 June (300 accumulated GDD), whereas peak emergence of adult P. formosus occurred the week of 3 July (678 accumulated GDD), approximately one month after P. impressifrons (Fig. 4A). In 2021, peak emergence of P. impressifrons occurred the week of 5 June (374 accumulated GDD), whereas peak emergence of P. formosus occurred seven days later, on 12 June (511 accumulated GDD; Fig. 4B).
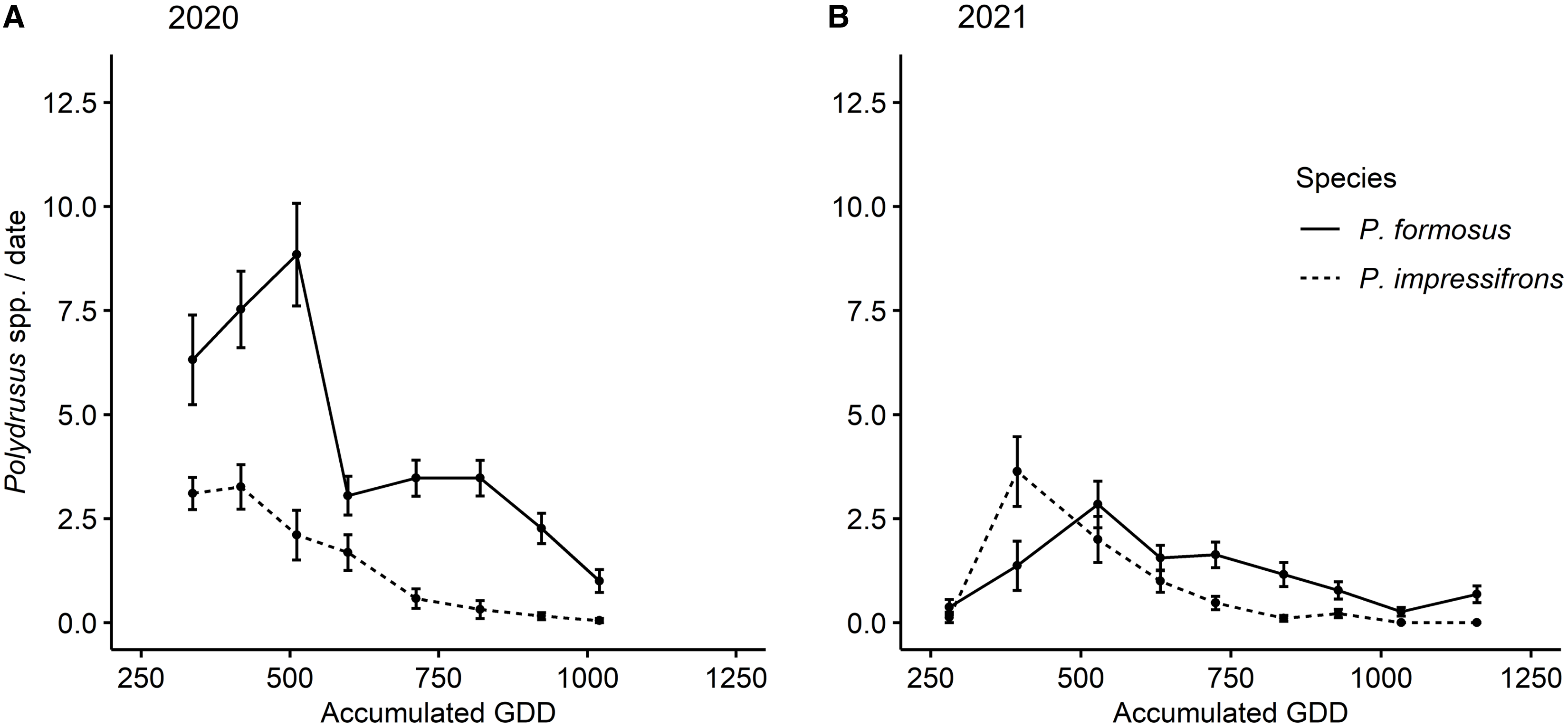
Figure 4. Mean abundance (± standard error) of nonnative adult Polydrusus per sampling (collected via beat-sheet samples) by accumulated growing degree days (GDD) throughout the season on hybrid hazel plants at Minnesota orchards in A, 2020 and B, 2021.
The abundance of the two species was significantly higher in 2020 than in 2021 (year: χ2 = 84.56, df = 1, P < 0.0001; species: χ2 = 91.35, df = 1, P < 0.0001; year × species interaction: χ2 = 2.68, df = 1, P = 0.102); therefore, years were analysed separately. In 2020, a maximum of 43 individuals (i.e., both species combined) were collected from a single plant on 27 May, as opposed to a maximum of 24 individuals collected from a single plant on 1 June 2021. In 2020, the season-long abundance of P. formosus per sampling event (i.e., per plant per sample date) was higher than that of P. impressifrons, with a mean of 2.01 individuals compared to 1.15, respectively, on any given plant (χ2 = 77.79, df = 1, P < 0.0001; Fig. 4). In 2021, the season-long abundance of P. formosus per sampling event was also higher than that of P. impressifrons, with a mean of 0.61 individuals compared to 0.43, respectively (χ2 = 9.86, df = 1, P = 0.0017; Fig. 4).
In 2020, the sex ratio of males to females was 1.3:1 (390:297 M:F) for P. formosus and 1.6:1 (239:154 M:F) for P. impressifrons. In contrast, in 2021, the sex ratio was 1.2:1 (105:88 M:F) for P. formosus and 1:1 (68:69 M:F) for P. impressifrons. Within each year, the sex ratios of the two species did not differ significantly from each other (2020: χ2 = 1.93, df = 1, P = 0.1653; 2021: χ2 = 0.63, df = 1, P = 0.4241). Sex ratios of P. formosus also did not differ between years, with 56.7% males in 2020 and 54.1% males in 2021 (χ2 = 0.42, df = 1, P = 0.5191). In contrast, sex ratios of P. impressifrons differed significantly between years, with a 60.9% male population in 2020 and a 49.6% male population in 2021 (χ2 = 5.33, df = 1, P = 0.0210).
Molecular identification
The DNA barcoding confirmed morphological identifications for both species. The specimen morphologically identified as Polydrusus formosus had a highest scoring match (100% identity, 100% coverage) to a CO1 sequence for a P. formosus specimen collected in Germany (GenBank accession number: KC784165.1). The specimen we morphologically identified as Polydrusus impressifrons resulted in a highest scoring match (98.26% identity, 60% coverage) to a CO1 sequence for a P. impressifrons specimen collected in Canada (GenBank accession number: KR490559.1). We additionally checked barcodes against the Barcode of Life Data System database and verified that these sequences binned with other confirmed P. formosus barcodes (BOLD: CO8630) and P. impressifrons (BOLD: O4332). Assembled CO1 sequences for both specimens were deposited in GenBank under accession numbers OQ451940 (P. formosus) and OQ451941 (P. impressifrons).
Discussion
Our results document the seasonal abundances, phenologies, and sex ratios of an established nonnative Polydrusus species complex on the same host plant. The phenology of P. formosus in hybrid hazelnut orchards is similar to previous findings in midwestern forests (Coyle et al. Reference Coyle, Mattson, Jordan and Raffa2012). Additionally, the photographic guide to morphological identification for all the Polydrusus species in the United States of America and Canada, their distributions, and how to distinguish between sexes of nonnative Polydrusus species will provide useful information for conservation, agriculture, and forest management efforts.
We first found adult P. formosus in the plantings in early June of both years, and their peak emergence was recorded in early July and mid-June for 2020 and 2021, respectively, which is consistent with findings of Coyle et al. (Reference Coyle, Mattson, Jordan and Raffa2012) in Michigan forests (Fig. 4). However, we found adult Polydrusus of both species in the hybrid hazel plants through early August, which is a longer period of adult activity than Coyle et al. (Reference Coyle, Mattson, Jordan and Raffa2012) noted for adult P. formosus. However, this phenomenon could be due to site differences in heat accumulation between southeastern Minnesota in 2020 and 2021 and the upper peninsula of Michigan in 2007–2009 (Coyle et al. Reference Coyle, Mattson, Jordan and Raffa2012). In addition, in contrast to our observed peak emergences for P. formosus, ranging from mid-June to early July depending on the year, Pinski et al.’s (Reference Pinski, Mattson and Raffa2005b) field-based study using flowerpots noted peak adult emergence for P. formosus in mid- to late May (Fig. 3). This earlier reported peak emergence for P. formosus from Pinski et al. (Reference Pinski, Mattson and Raffa2005b) is likely due to rearing conditions, because in the spring soil warms much faster in flowerpots than in ground (Ingram et al. Reference Ingram, Ruter and Martin2015).
Our research is the first to compare the relative seasonal abundances of adult P. formosus and P. impressifrons on the same host plants. In both years, we observed higher mean seasonal abundances of adult P. formosus than of adult P. impressifrons on hybrid hazels (Fig. 4), with more P. formosus than P. impressifrons on any given plant throughout the seasons in both 2020 and 2021. This finding could be due to several reasons, including differential species performance at northern latitudes or on different host plants. For example, P. formosus has been documented historically more often on hazel than P. impressifrons has and has more occurrence records for the upper Midwest region of North America (Pierce Reference Pierce1916; Frost Reference Frost1946; Morris Reference Morris1978; Casteels and de Clercq Reference Casteels and de Clercq1988; Fig. 2C, D). Our finding could also reflect interspecific competition or the formation of a pest complex, where the two species combined induce more damage to a host plant than each species alone. However, we did not record survival of the Polydrusus species over their life cycles or potential displacement that would allow us to confirm these conjectures.
We observed fewer individuals for both Polydrusus species throughout the season in 2021 than in 2020. This finding could be due to the drought that both sites experienced in the summer of 2021 that decreased crop yields in the state (Davenport et al. Reference Davenport, Kreiter, Brauman, Keeler, Arbuckle and Sharma2022). Drought can have detrimental effects on root-feeding insects, including the eggs and larvae of the clover root weevil, Sitona lepidus Gyllenhaal (Coleoptera: Curculionidae), a weevil within Entiminae, the same subfamily as Polydrusus (Johnson et al. Reference Johnson, Gregory, McNicol, Oodally, Zhang and Murray2010). We observed more male P. impressifrons than females in 2020 but no difference in sexes in 2021. The presence of more males than females in 2020 could be due to increased overwintering success for males in that year (Dix and All Reference Dix and All1985).
Despite the abundance of Polydrusus observed in our plantings and their negative impacts in other tree systems (Pierce Reference Pierce1916; Nordman et al. Reference Nordman, Robison, Abrahamson and Volk2005; Rodstrom Reference Rodstrom2013; Niedbala et al. Reference Niedbala, Rodstrom and Brown2017), we did not observe noticeable insect defoliation on the foliage of hybrid hazel plants in our orchards besides that from Popillia japonica (Newman) (Coleoptera: Scarabaeidae), which is easily recognisable (Potter and Held Reference Potter and Held2002; Shanovich et al. Reference Shanovich, Dean, Koch and Hodgson2019). However, recording above-ground defoliation by adult Polydrusus weevils was not an objective of this study, and any damage may have been missed. The abundance of P. formosus and P. impressifrons that we observed throughout the season in our orchards suggests feeding by the weevils is occurring on the foliage, but it is unclear if they are decreasing plant vigour or yields. Tree plantations and orchard systems can be severely affected belowground by rhizophagous insects, reducing the survival of saplings and tree yields (Kard and Hain Reference Kard and Hain1987; Thakur Reference Thakur1988; Meshram et al. Reference Meshram, Pathak and Jamaluddin1990; Wagner et al. Reference Wagner, Tobi, Wallner and Parker1991; Leather et al. Reference Leather, Day and Salisbury1999; Borowicz et al. Reference Borowicz, Alessandro, Albrecht and Mayer2005; Duncan et al. Reference Duncan, Stuart, El-Borai, Campos-Herrera, Pathak, Giurcanu and Graham2013). Large numbers of P. impressifrons have been known to emerge after the rotation of a poplar planting (i.e., after the harvest of mature trees), when newly regenerating sprouts from coppiced trees or newly propagated young trees provide the only food source (Niedbala et al. Reference Niedbala, Rodstrom and Brown2017). Hybrid hazelnut production also uses a rotational coppice system after plants surpass peak nut production, and young plants may be at similar risk in places (Braun and Jensen Reference Braun and Jensen2015). Lastly, hybrid hazelnut orchards bordered by woods containing suitable host plants may be at higher risk to colonisation from nonnative Polydrusus species. Indeed, adults of P. impressifrons are noted to be strong fliers with the ability to recolonise plantings throughout the summer (Niedbala et al. Reference Niedbala, Rodstrom and Brown2017; Supplementary material, Table S1). Future research is needed to illuminate the impact of Polydrusus species, both above and below ground, on woody perennial crops such as hazel.
We found no native Polydrusus species during our sampling over the two years in hybrid hazelnut orchards. However, members of the birch family are not noted to be host plants of any of the Polydrusus species native to North America (Supplementary material, Table S1). Overall, there are very few records and observations of native Polydrusus species throughout the United States of America and Canada compared to nonnative species (Fig. 2). In a field or orchard setting, such as a hazel planting, populations of nonnative weevil species could increase at faster rates in the potential absence of associated forest-dwelling natural enemies and other forest-associated habitat characteristics and reach a level of concern where pest management is required. However, it is unclear if native Polydrusus are being displaced by nonnative Polydrusus, as Coyle et al. (Reference Coyle, Mattson, Raffa, Johnson and Murray2008) suggest, or if the native species are rare to begin with.
The compendium of species descriptions we compile here is comprised of high-quality photographs, which will make species and sex identification of the Polydrusus species of the United States of America and Canada more accessible, precluding the need to search across multiple taxonomic monographs. Because nonnative Polydrusus species may be displacing or outcompeting native species, it is important for researchers to be able to distinguish between them in order to monitor their conservation status.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.4039/tce.2024.7
Acknowledgements
The authors thank the following: Lois Braun and Mark Hamann, University of Minnesota, for the use of the experimental hybrid hazel orchards and site maintenance; Pheylan Anderson, Alexa Koch, and Patrick Perish for their assistance with data collection and curation; Dr. Daniel Cariveau, University of Minnesota, for consultation on statistical analyses; and the University of Minnesota Insect Collection for access to Polydrusus specimens for identification purposes. The authors also thank Patrick J. Leisch, director of the insect diagnostic laboratory at the University of Wisconsin – Madison, for confirmation of the authors’ specimen determinations and for the deposition of specimens into the Wisconsin Insect Research Collection, with help from Collection curator Dr. Craig Brabant. This work was supported by funds from the United States Department of Agriculture – Specialty Crop Research Initiative (Award 2019-51181-30025), the Louise Dosdall Fellowship from the University of Minnesota Graduate School awarded to HNS in 2022, and a research award from the James. W. Wilkie Fund for Natural History of the Bell Museum to HNS in 2022. The authors acknowledge that this research took place on the traditional, ancestral, and contemporary lands of the Očhéthi Šakówiŋ Oyate (i.e., Dakota peoples), which hold great historical, spiritual, and personal significance for its original stewards.









