Insulin resistance (IR) has been accepted as a key factor in the development of impaired glucose tolerance, type 2 diabetes mellitus (Bonora et al., Reference Bonora, Kiechl, Willeit, Oberhollenzer, Egger, Meigs and Muggeo2004; Hanley et al., Reference Hanley, Williams, Gonzalez, D'Agostino, Wagenknecht, Stern and Haffner2003; Hayashi et al., Reference Hayashi, Boyko, Leonetti, McNeely, Newell-Morris, Kahn and Fujimoto2003; Song et al., Reference Song, Manson, Tinker, Howard, Kuller, Nathan and Liu2007), cardiovascular disease (Gast et al., Reference Gast, Tjeerdema, Stijnen, Smit and Dekkers2012), and even cancer (Tsugane & Inoue, Reference Tsugane and Inoue2010). Besides obesity (Kahn et al., Reference Kahn, Hull and Utzschneider2006) and low birth weight (Grunnet et al., Reference Grunnet, Vielwerth, Vaag and Poulsen2007; Skidmore et al., Reference Skidmore, Cassidy, Swaminathan, Richards, Spector and MacGregor2008), weight gain regardless of weight status at baseline has been independently associated with higher IR (Chang et al., Reference Chang, Sung, Yun, Jung, Kim, Kwon and Ryu2013; Everson et al., Reference Everson, Goldberg, Helmrich, Lakka, Lynch, Kaplan and Salonen1998; Lakka et al., Reference Lakka, Salonen, Tuomilehto, Kaplan and Lakka2002). However, it remains uncertain whether the relationship is a genetic effect or of non-genetic origin. To differentiate the genetic or non-genetic impact on the association between low birth weight and IR, studies have investigated the relationship in discordant monozygotic twins to eliminate genetic effects and demonstrate non-genetic mechanisms (Grunnet et al., Reference Grunnet, Vielwerth, Vaag and Poulsen2007; Skidmore et al., Reference Skidmore, Cassidy, Swaminathan, Richards, Spector and MacGregor2008; Vaag & Poulsen, Reference Vaag and Poulsen2007) By comparison, the knowledge of genetic and non-genetic influences on the relationship between weight change in adult life and IR seems to be limited. In other words, a genetically determined, inappropriate insulin secretion and action may result in both weight change and susceptibility to IR. Otherwise, environmental factors such as lifestyle may influence the two phenotypes. Investigating the relationship between weight change from 20 years of age and IR in monozygotic twins allows a unique opportunity to assess the mediation of non-genetic factors in the relationship.
This study aimed to examine the genetic and environmental relationships between weight change from the age of 20 years and IR traits using four indicators — fasting plasma glucose, fasting plasma insulin, the homeostasis model assessment of IR (HOMA-IR), and the quantitative insulin sensitivity check index (QUICKI) — in a population-based cohort of twins and their family members.
Subjects and Methods
Subjects and Study Design
The study subjects comprised a sample of twins and their family members enrolled in the Healthy Twin Study, which has been conducted as a part of the Korean Genomic Epidemiologic Study since 2005. Participants typically included same-sex twin pairs and their first-degree family members. A previous study described the design and selection criteria of the Healthy Twin Study in detail (Sung et al., Reference Sung, Cho, Lee, Ha, Choi, Choi and Song2006). The current study included 594 individuals (209 men [45.3 ± 11.6 years of age], 385 women [43.4 ± 10.3 years of age]; 240 monozygotic [38.3 ± 6.4 years of age] and 53 dizygotic [38.9 ± 6.9 years of age] twin individuals, and 301 non-twin family members [41 fathers, 66 mothers, and 194 siblings, 49.5 ± 11.4 years of age] from 192 families) who had complete data for self-reported body weight at the age of 20 years, measured bodyweight and height, and fasting plasma glucose and insulin, from 2009 to 2010. None had previous diabetes mellitus. Zygosity of twins was determined using a questionnaire that achieved greater than 90% accuracy and genetic analysis using 16 short tandem repeat markers (15 autosomal-markers and one sex-determining marker, AmpFlSTR Identifier Kit; Perkin Elmer, Waltham, MA, USA; Song et al., Reference Song, Lee, Lee, Lee, Lee, Hong and Sung2010) All study procedures were approved by the institutional review boards of the participating institutions.
Clinical and Biochemical Measurements
The measurements of weight and height were made with the subjects wearing indoor clothing and were without shoes. Bodyweight and standing and seated height were measured twice to the nearest 0.1 kg and 0.1 cm, respectively, using a digital balance (Tanita Co., Seoul, Korea) and a stadiometer (Samwha Co., Seoul, Korea). The body mass index (BMI) was calculated as the weight (in kilograms) divided by the height squared (m2). Venous blood was drawn after an overnight fast and analyzed in a central laboratory that was authorized by the Korea Association of Quality Control over the Clinical Laboratory Examination. Plasma glucose was assayed using a hexokinase enzymatic method and plasma insulin was determined by immunoradiometric assay using ADVIA 1650 (Siemens, Germany) or HITACHI 7600-210/HITACHI 7180 (HITACHI, JAPAN). The HOMA-IR, an estimate of basal insulin sensitivity, was calculated using the following formula: fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5 (Matthews et al., Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner1985; Wallace et al., Reference Wallace, Levy and Matthews2004). The QUICKI, a robust index of insulin sensitivity, was computed as follows: 1/[log(fasting insulin μIU/mL) + log(fasting glucose mg/dL)] (Katz et al., Reference Katz, Nambi, Mather, Baron, Follmann, Sullivan and Quon2000). Interassay coefficient of variation for glucose and insulin measurement was set below 7%.
A self-administered standard questionnaire was used to collect information about demographic characteristics, weight at 20 years of age, alcohol use (user vs. non-user), cigarette smoking (non-smoker vs. ever-smoker), physical activity, and dietary intake. Physical activity was assessed using the Korean version of the International Physical Activity Questionnaire (Craig et al., Reference Craig, Marshall, Sjostrom, Bauman, Booth, Ainsworth and Oja2003) and daily caloric intake was evaluated using a validated 103-item semi-quantitative food frequency questionnaire (Ahn et al., Reference Ahn, Lee, Cho, Shin, Park, Oh and Kim2004).
Statistical Analysis
The percentage of change in weight was computed as follows: 100 × [measured weight at study enrollment (kg) — self-reported weight at age (kg)]/self-reported weight at the age of 20 years (kg)]. The percentage of weight change was categorized into four even quartile groups. The demographic characteristics, lifestyle characteristics (alcohol use, smoking status, physical activity, and dietary intake) and IR traits were compared by weight change groups using ANOVA (and post hoc comparison using the Scheffe test if there was a significant difference between groups and polynomial contrast test for a linear trend) or the chi-square test (and linear-by-linear association test for a trend). The relationships between weight change (as the quartiles or a continuous variable) and IR traits were analyzed using a linear mixed model, in which the correlation structures from family relationships were considered by adjusting for household and twin effect as a random effect, and other covariates (sex, age, BMI at the age of 20 years, and lifestyle) were adjusted as fixed effects. This analysis was also conducted for the relationships between two IR traits (HOMA-IR and QUICKI) and the combined weight groups (combinations of quartiles of percentage of weight change and quartiles of BMI at the age of 20 years). To evaluate whether the associations between weight change and IR traits were of non-genetic origin, Spearman correlations were applied for the phenotypic correlations between within-pair differences in percentage of weight change and within-pair differences in IR traits in 98 pairs of monozygotic twins. In addition, we conducted a co-twin control study in 55 pairs of monozygotic twins who were discordant for level of HOMA-IR by 0.3. Within-pair comparison of percentage of weight change and BMI at the age of 20 years and current BMI were conducted using paired t tests. The risk for having a greater level of HOMA-IR associated with weight change, BMI at the age of 20 years, and current BMI was assessed using conditional logistic regression analysis with an adjustment for lifestyle factors, in which a subject who had a higher level of HOMA-IR than his/her co-twin was compared with the co-twin. The significance level was determined as p < .05. These analyses were performed using PASW, Statistics 18 (SPSS, Chicago, IL, USA).
The genetic analysis was conducted using the Sequential Oligogenic Linkage Analysis Routines (SOLAR) package (http://solar.sfbrgenetics.org, version 6.6.2). Heritability of weight change and IR traits were calculated as the proportion of phenotypic variance explained by additive genetic effects with adjustment for age and sex. To examine the evidence of common genetic and environmental regulation between weight change and IR traits, we partitioned the phenotypic correlations into correlations explained by genetic sharing (ρG) and environmental sharing (ρE), after adjusting for age, sex, alcohol use, smoking status, and BMI at 20 years old. If ρG or ρE significantly deviated from zero, we regarded it as evidence of a genetic or environmental association between the two traits, respectively.
Results
In Table 1, the distribution of IR traits and demographic, anthropometry, and lifestyle-related characteristics were compared according to the quartiles of percentage of weight change from 20 years of age. The higher quartile subgroups of weight change were more likely to be men, have a lower BMI at the age of 20 years, and have higher glucose, insulin, and HOMA-IR and lower QUICKI.
TABLE 1 The Comparison of Characteristics Between Quartiles of Percentage of Weight Change From the Age of 20 Years
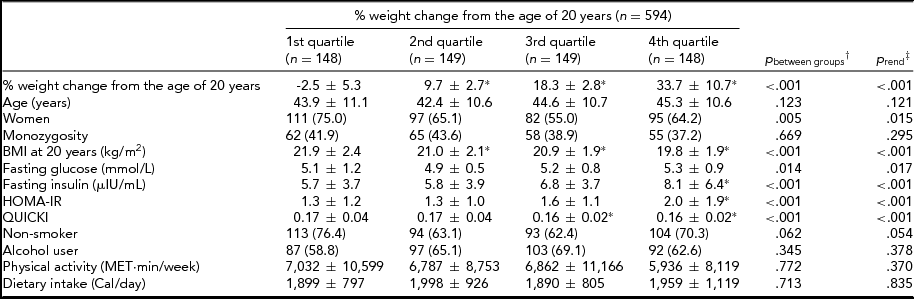
Values were mean ± SD or N (%).
% weight change from the age of 20 years = [(measured weight-reported weight at 20 years) × 100/reported weight at 20 years].
BMI = body mass index; HOMA-IR = Homeostasis Model Assessment of Insulin Resistance Index; QUICKI = Quantitative Insulin Sensitivity Check Index.
*p < .05 compared to 1st quartile group using Scheffe test (post-hoc analysis).
†ANOVA or Pearson chi-square tests.
‡Polynomial test of ANOVA or linear-by-linear association tests.
As shown in Table 2, higher quartiles of percentage of weight change were associated with increases in insulin and HOMA-IR and a decrease in QUICKI after adjustment for age, sex, household and twin effects, and lifestyle (p for trend < .05). The associations were persistent and even the association with glucose became significant with further adjustment with BMI at the age of 20 years. However, all associations between weight change and IR traits were not more significant when current BMI was adjusted instead of BMI at the age of 20 years.
TABLE 2 The Associations Between Insulin Resistance Traits and Percentage of Weight Change From the Age of 20 Years
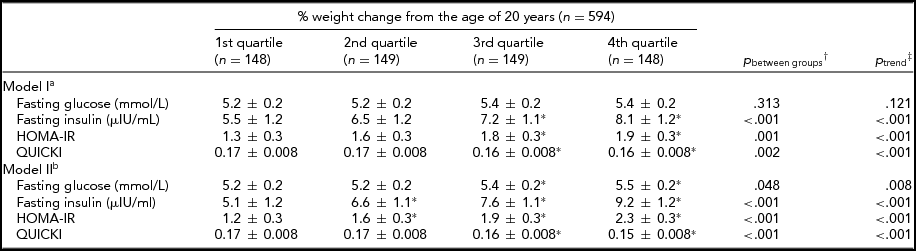
Values were estimated mean ± SE.
% weight change from the age of 20 years = [(measured weight-reported weight at 20 years) × 100/reported weight at 20 years].
HOMA-IR = Homeostasis Model Assessment of Insulin Resistance Index; QUICKI = Quantitative Insulin Sensitivity Check Index.
*p < .05 compared to 1st quartile group using †simple contrast test of linear mixed model.
‡Polynomial contrast test of linear mixed model.
aAdjusted for sex, age, smoking status, alcohol use, physical activity, calorie intake as random effects and twin and household effects as fixed effects.
bAdjusted for body mass index at 20 years and covariates of model I.
Figure 1 depicts the associations with HOMA-IR and QUICKI regarding the combination of quartiles of percentage of weight change from the age of 20 years and quartiles of BMI at the age of 20 years. The HOMA-IR increased with higher quartiles of weight change in the 2nd (p for trend = .009), 3rd (p for trend = .012), and 4th (p for trend = .041) quartiles of BMI at the age of 20 years. Likewise, QUICKI decreased with higher quartiles of weight change in the 3rd quartile of BMI at the age of 20 years (p for trend = .004).
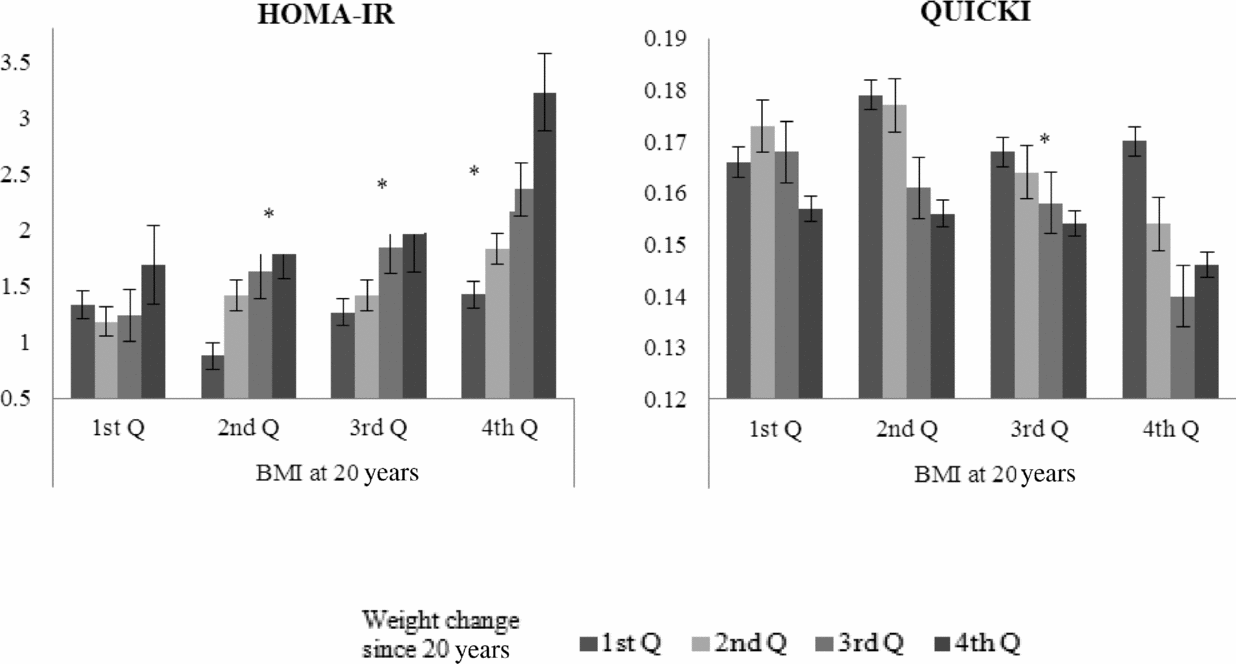
FIGURE 1 The estimated mean (standard error, SE) of the homeostasis model assessment of insulin resistance (IR) index (homeostasis model assessment of IR, HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI) according to combination of quartiles of weight change since 20 years of age and quartiles of body mass index (BMI) at the age of 20 years. Note: *p for trend <.05 using linear mixed model adjusted for fixed effects (age, sex, smoking, alcohol use, physical activity, caloric intake, and BMI at 20 years old) and random effects (twins and household effects).
Table 3 presents the phenotypic relationships and cross-trait correlations between percentage of weight change and IR traits. With a 1% weight gain from 20 years of age, glucose, insulin, and HOMA-IR increased by 0.0097 mmmol/L, 0.11 μIU/mL, and 0.028, respectively, while QUICKI decreased by 0.0006. Estimated heritability was highest for glucose, followed by insulin, HOMA-IR, and QUICKI (p < .001). The estimated heritability ± SE (standard error) for percentage of weight change from 20 years of age was 0.587 ± 0.06 (p < .001). In cross-trait correlations, the phenotypic correlations were stronger for insulin, HOMA-IR, and QUICKI than for glucose. When the correlations were further partitioned into genetic and environmental components, the environmental correlations were higher as compared to the genetic correlations.
TABLE 3 Associations and Cross-Trait Correlations Between Percentage of Weight Change From the Age of 20 Years and Insulin Resistance Index
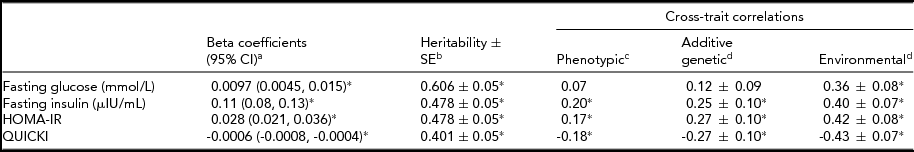
% weight change from the age of 20 years = [(measured weight-reported weight at the age of 20 years) × 100/reported weight at the age of 20 years].
HOMA-IR = Homeostasis Model Assessment of Insulin Resistance Index; QUICKI = Quantitative Insulin Sensitivity Check Index; CI = confidence interval; SE = standard error.
*p < .05.
aLinear mixed model adjusted for fixed effects (age, sex, smoking, alcohol use, physical activity, calorie intake, and BMI at 20 years old) and a random effect (household).
bAdjusted for age and sex.
cPartial correlation coefficients after adjusted for age, sex, smoking, alcohol use, physical activity, calorie intake, and BMI at 20 years.
dEstimate ± SE were assessed by bivariate analysis after adjusted for age, sex, smoking, alcohol use, and BMI at 20 years.
In 98 pairs of monozygotic twins, the within-pair difference in percentage of weight change from the age of 20 years was correlated significantly with within-pair differences in fasting insulin and HOMA (p < .05) among the IR traits (Table 4). In 55 pairs of monozygotic twins who were discordant for the level of HOMA-IR by >0.3, conditional logistic regression analyses show that the odds ratio (95% confidence interval) for having a higher level of HOMA-IR was 1.10 (1.03–1.17) with a 1% increase in weight change from the age of 20 years and 2.15 (1.39, 3.34) with a 1 kg/m2 increase in current BMI after adjusting for lifestyle-related factors (Table 5).
TABLE 4 Spearman Correlations Between Within-Pair Difference in Percentage of Weight Change From the Age of 20 Years and Within-Pair Differences in Insulin Resistance Traits in 98 Monozygotic Twins
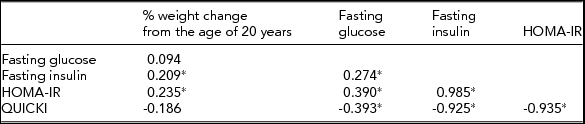
% weight change from the age of 20 years = [(measured weight at initial visit-reported weight at the age of 20 years) × 100/reported weight at the age of 20 years].
HOMA-IR = the Homeostasis Model Assessment of Insulin Resistance Index; QUICKI = Quantitative Insulin Sensitivity Check Index.
*p < .05.
TABLE 5 Risk Estimation for Having Higher Level of HOMA-IR: Co-Twin-Control Analysis in 55 Pairs of Monozygotic Twins Whose HOMA Levels Differ Each Other By More Than 0.3

% weight change from the age of 20 years = [(measured weight at initial visit-reported weight at the age of 20 years) × 100/reported weight at the age of 20 years].
HOMA-IR = Homeostasis Model Assessment of Insulin Resistance Index; SD = standard deviation; CI = confidence interval.
a Paired t test.
bConditional logistic regression analysis after adjusted for alcohol use, smoking status, physical activity, and calorie intake.
Discussion
Identifying factors related to IR is important because of its association with higher risk for type 2 diabetes (Bonora et al., Reference Bonora, Kiechl, Willeit, Oberhollenzer, Egger, Meigs and Muggeo2004; Hanley et al., Reference Hanley, Williams, Gonzalez, D'Agostino, Wagenknecht, Stern and Haffner2003; Hayashi et al., Reference Hayashi, Boyko, Leonetti, McNeely, Newell-Morris, Kahn and Fujimoto2003; Song et al., Reference Song, Manson, Tinker, Howard, Kuller, Nathan and Liu2007), cardiovascular disease (Gast et al., Reference Gast, Tjeerdema, Stijnen, Smit and Dekkers2012), cancer (Tsugane & Inoue, Reference Tsugane and Inoue2010), and all-cause mortality (Ausk et al., Reference Ausk, Boyko and Ioannou2010). In this study of Korean twins and their family members, we found greater weight gain (and consequently higher current BMI) from the age of 20 years to be an independent factor associated with higher IR and lower insulin sensitivity, regardless of their current lifestyle and BMI at the age of 20. Despite inconsistent definitions for IR between studies, our findings are consistent with previous results (Chang et al., Reference Chang, Sung, Yun, Jung, Kim, Kwon and Ryu2013; Everson et al., Reference Everson, Goldberg, Helmrich, Lakka, Lynch, Kaplan and Salonen1998; Lakka et al., Reference Lakka, Salonen, Tuomilehto, Kaplan and Lakka2002). Several mechanisms could explain these associations. The deleterious effects of adipocyte in obesity on IR and insulin sensitivity have been well established. Weight gain could increase adipose tissue. Excess adiposity increases the release of adipocyte-derived factors such as non-esterified fatty acids and proinflammatory cytokines as well as other products such as tumor necrosis factor-α, interleukin-6, monocyte chemoattractant protein-1, and additional products of macrophages and other cells (Kahn et al., Reference Kahn, Hull and Utzschneider2006; Shoelson et al., Reference Shoelson, Lee and Goldfine2006). The interaction between genes and environment could play a role in inducing weight change and IR. Several genes and genetic mutations associated with insulin sensitivity and obesity have been identified (Barroso, Reference Barroso2005; Kahn et al., Reference Kahn, Hull and Utzschneider2006). Moreover, environmental factors producing long-term caloric imbalance such as excess caloric intake and low physical activity could be responsible for the association. Current findings regarding genetic and environmental correlations between weight change and IR traits support these mechanisms to some extent. In addition, a significant non-genetic effect on these relationships found in the co-twin control study highlights the importance of environment in this relationship.
The studies examined for genetic and environmental contribution in the relationship between weight change in adulthood and IR are very limited. Skidmore et al. (Reference Skidmore, Cassidy, Swaminathan, Richards, Spector and MacGregor2008) reported that the relationship between BMI as a consequence of weight change and IR was not mediated through genetic factors in 1,194 female twins aged from 18 to74 years; however, there were significant within- and between-pair differences in the relationship, which implies both individual-specific and shared environmental effects in twins explained the relationship. In our co-twin-control study design, which completely adjusts age, sex, genetic influence, and potentially shared environmental effect, we were not able to examine genetic effect in the relationship; however, the significant genetic correlations between weight change and IR traits, except for fasting plasma glucose, may suggest the potential of genetic pleiotropic effects on weight change and IR traits.
There are some potential limitations in our study. First, to our knowledge, as there are no comparable studies on the genetic and environmental relationships between weight change and IR traits, we could not directly compare current findings with the previous ones. Second, weight at 20 years of age was assessed by self-report and may not reflect accurate weight because of the potential of over- and under-estimation. However, several studies have indicated that self-reported weight during young adulthood can be used in epidemiologic research (Perry, Reference Perry, Byers, Mokdad, Serdula and Williamson1995; Stevens et al., Reference Stevens, Keil, Waid and Gazes1990). Third, we were not able to examine which individual body component influences the apparent associations between weight change from the age of 20 years and IR traits. Probably, changes in subcutaneous adipose tissues and visceral adipose tissue would have different impact on insulin resistance and insulin sensitivity (Pourhassan et al., Reference Pourhassan, Bosy-Westphal, Schautz, Braun, Gluer and Muller2014). Finally, due to lack of information for IR traits at the age of 20 years, we were not able to evaluate the potential effect of IR at the age of 20 years on the current IR (Everson et al., Reference Everson, Goldberg, Helmrich, Lakka, Lynch, Kaplan and Salonen1998). However, a temporal relationship such as the precedence of weight change before IR could be plausible, although we obtained the information for weight at the age of 20 years and IR traits cross-sectionally.
Despite these limitations, this co-twin-control study allows for control of genetic factors, and common environmental and maternal factors with a putative influence on higher IR in monozygotic twins (Vaag & Poulsen, Reference Vaag and Poulsen2007), and then demonstrates evidence for non-genetic associations between IR and weight change from the age of 20 years; however, as we cannot indicate a specific pathway to explain the mediation of non-genetic effect in the relationship between weight change and IR traits, further research is warranted to demonstrate specific environmental factors such as nutrient composition and food pattern (Brand-Miller et al., Reference Brand-Miller, McMillan-Price, Steinbeck and Caterson2009; Buscemi et al., Reference Buscemi, Nicolucci, Mattina, Rosafio, Massenti, Lucisano and Rini2013).
In conclusion, in this Korean twin and family study, we demonstrated that phenotypic associations between weight change from the age of 20 years and IR traits, and the genetic and environmental contribution to the associations. Given the limited evidence for genetic and environmental influences on this link, further investigation may provide crucial genetic and environmental pathway for the relationship between weight change and IR.
Acknowledgments
This study was supported by the National Genome Research Institute, Korea, National Institute of Health research contract (budgets 2011E7101100, 2012E7100200), and National Research Foundation of Korea (NRF-2013R1A1A2057608). The views expressed in this article are those of the authors and not any funding body.










