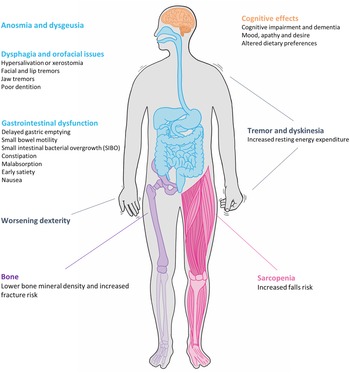
Parkinson's disease (PD) is the second most common neurodegenerative condition with an estimated prevalence of 1 % in those over 60 years(Reference Parkinson1,Reference de Lau and Breteler2 ). First described in 1817 in ‘An Essay on the Shaking Palsy’, James Parkinson described both the motor and non-motor features of the condition. Parkinsonism is a symptom complex consisting of akinesia, rigidity, tremor and postural instability, the latter of which tends to emerge as the disease progresses. The onset of motor signs is often pre-dated by non-motor symptoms which encompass neuropsychiatric, sleep and autonomic dysfunction(Reference Chaudhuri, Healy and Schapira3). Nutrition is an often overlooked but important factor in the management of the disease; dysfunction is invariably multifactorial in aetiology, different features emerge over the disease course and vary in the degree to which they impact quality of life (Fig. 1).

Fig. 1. Nutrition-related issues faced by patients with Parkinson's disease.
Idiopathic PD is the commonest cause of parkinsonism and is diagnosed clinically, usually with at least two of the four clinical signs in accordance with the UK Queens Square Brain Bank criteria(Reference Hughes, Daniel and Kilford4). Imaging has a supportive role particularly where features such as early falls, lack of response to levodopa, lack of tremor, rapid progression or dysautonomia raise the possibility of an alternative diagnosis(Reference Suchowersky, Reich and Perlmutter5). The mainstay of treatment is focused on compensating for the primary loss of dopamine that results from presynaptic degeneration of dopaminergic cells in the substantia nigra and striatum. Dopaminergic drugs offer a gratifying response, particularly early in the disease. The heterogeneity of the disease is such that the choice of treatment regimen is highly individualised.
Hoehn and Yahr described five levels of clinical disability in PD from stage 1, in which there is unilateral involvement and minimal or no functional impairment, through to stage 5, in which the individual is confined to bed/wheelchair(Reference Hoehn and Yahr6). Although these stages may not correlate with pathophysiological stages, this scale aims to have practical utility and be reproducible when scored by different clinicians. The Movement Disorder Society-Unified Parkinson's Disease Rating Scale, which evaluates motor signs, the impact of motor and non-motor symptoms on daily living and motor complications, is the most widely used clinical rating scale for PD(Reference Goetz, Tilley and Shaftman7). Macmahon and Thomas proposed a scale to describe the clinical course of PD from the ‘diagnostic’ phase; through a relatively stable ‘maintenance’ phase; progressing to a ‘complex’ phase, characterised by motor fluctuations, development of cognitive impairment and appearance of axial symptoms, including falls, freezing of gait and dysphagia; finally reaching a palliative phase(Reference MacMahon and Thomas8). It is now recognised that several symptoms, in particular rapid-eye-movement sleep behaviour disorder, constipation, anosmia and depression, known as premotor symptoms, may precede the onset of motor symptoms by many years, during what is referred to as the ‘prodromal’ phase(Reference Schrag, Horsfall and Walters9,Reference Kaiserova, Grambalova and Kurcova10) .
Pathophysiology and aetiology of Parkinson's disease
PD is characterised pathologically by the loss of dopaminergic neurons in the substantia nigra, thought to be due to the accumulation within neurons of aggregated forms of the protein α-synuclein, known as Lewy bodies. By studying post-mortem brains from individuals with and without prior symptoms of parkinsonism, Braak and colleagues described a sequential progression of neuronal damage throughout the nervous system, categorised into six neuropathological stages: stage 1 consisting of lesions in the dorsal motor nucleus of the glossopharyngeal and vagal nerves, which later progress to reach the brainstem, finally involving the cerebral cortex in stage 6(Reference Braak, Del Tredici and Rüb11). Crucially, the hallmark lesions of PD were noted to develop before the clinical appearance of motor and non-motor dysfunction(Reference Braak, Del Tredici and Rüb11). In a subsequent neuropathological study, Braak and colleagues identified Lewy bodies within the myenteric and submucosal plexuses, the collections of neurons which comprise the enteric nervous system which controls the function of the gastrointestinal (GI) tract(Reference Braak, de Vos and Bohl12). This has led to the suggestion that a neurotropic pathogen, which triggers PD pathology, may enter the central nervous system via a nasal and gastric route; the so-called ‘dual-hit hypothesis’(Reference Hawkes, Del Tredici and Braak13).
A minority of patients with PD have a Mendelian form of the disease, such that the disease is caused by the inheritance of a single causative gene (e.g. SNCA, LRRK2, PARK2 and PINK1) in either an autosomal dominant or recessive pattern(Reference Lill, Roehr and McQueen14). However, such cases of familial parkinsonism are rare and, in most cases, PD is believed to be caused by a combination of genetic and environmental factors. Genome-wide association studies look for variability across the genome to determine whether any identified SNPs occur at a differing frequency between PD cases and controls(Reference Vázquez-Vélez and Zoghbi15). Multiple loci have been potentially implicated in PD(Reference Lill, Roehr and McQueen14); these low penetrance variants, which each have only a minimal impact on risk, may collectively increase an individual's risk(Reference Vázquez-Vélez and Zoghbi15). Similarly, numerous observational studies have examined the impact of environmental factors on the risk of PD, including dietary factors such as alcohol consumption and caffeine intake; exposure to environmental toxins, such as pesticides; biomarkers, such as BMI; drugs; comorbid illness and lifestyle factors including smoking(Reference Bellou, Belbasis and Tzoulaki16). However, apparent associations may not reflect a causal relationship due to potential residual confounding and reverse causation. There may also be interactions between genetic and environmental risk factors such that the impact of any environmental factors is modified by the background genetic risk(Reference Jacobs, Belete and Bestwick17).
In this review, we will examine nutrition in PD across three domains: dietary intake and the development of PD; whole body metabolism in PD including an overview of energy balance and musculoskeletal health and the effects of PD symptoms and treatment on nutritional status.
Dietary intake and the development of Parkinson's disease
Single nutrients
Oxidative stress plays a role in the development of PD(Reference Trist, Hare and Double18–Reference Puspita, Chung and Shim20). Antioxidant vitamins such as vitamins A, C, E and β-carotene have established roles in reducing cell damage from free radicals, and there has been much interest in whether higher intake of these nutrients reduces PD risk. Data from the Health Professionals Follow-Up Study and the Nurses' Health Study cohorts suggest lower PD risk for the highest v. the lowest quintile of dietary vitamin E(Reference Zhang, Hernán and Chen21), although at later follow-up, no association was found with vitamins C, E or carotenoids(Reference Hughes, Gao and Kim22). In two Swedish cohorts, higher dietary β-carotene intake was associated with lower PD risk, and there was also an inverse association with vitamin C and E intake but only in women(Reference Yang, Wolk and Håkansson23). Two meta-analyses suggest a neuroprotective effect of dietary vitamin E but not vitamins C and A or β-carotene(Reference Etminan, Gill and Samii24,Reference Takeda, Nyssen and Syed25) , although these predate more recent findings from the Health Professionals Follow-Up Study, the Nurses' Health Study and the Swedish cohort studies. Elevated plasma homocysteine has been described in neurodegenerative disorders(Reference Selhub26,Reference Coppedè27) and evidence suggests that polymorphisms in C1 metabolism may increase PD risk(Reference Murray and Jadavji28). Due to the importance of B vitamins in C1 metabolism(Reference Selhub26), B6, folate and B12 have been investigated prospectively in the context of PD risk(Reference de Lau, Koudstaal and Witteman29,Reference Chen, Zhang and Schwarzschild30) although the findings of these studies have been inconclusive.
The potential association between vitamin D intake and status has been explored with respect to PD risk (Reference Knekt, Kilkkinen and Rissanen31,Reference Larsson, Singleton and Nalls32) and progression(Reference Evatt, Delong and Khazai33,Reference Ding, Dhima and Lockhart34) outwith of its established roles in musculoskeletal health(Reference Hiller, Murchison and Lobb35). The literature in relation to vitamin D and the risk of developing PD has been distorted by several studies(Reference Sato, Honda and Iwamoto36–Reference Sato, Honda and Kaji40), which, prior to retraction, influenced widely cited reviews and meta-analyses(Reference Hiller41). A Finnish cohort study reported an inverse relationship between serum 25-hydroxyvitamin D (25(OH)D) and PD risk in those in the highest and lowest quartiles(Reference Knekt, Kilkkinen and Rissanen31). In contrast, a Mendelian randomisation study reported a lack of support for a causal association between low 25(OH)D and risk of PD(Reference Larsson, Singleton and Nalls32). Although there is limited evidence that vitamin D is protective against the development of PD(Reference Fullard and Duda42), low serum 25(OH)D in those with established PD may partially explain poorer musculoskeletal health(Reference Zhang, Zhang and Mao43).
Macronutrients such as dietary fat and its individual fatty acids may influence PD risk, although there is significant heterogeneity in the literature(Reference Chen, Zhang and Hernán44–Reference Tan, Methawasin and Tan49). Three meta-analyses, each with a different focus, investigated the relationship between dietary fat and the risk of developing PD(Reference Kamel, Goldman and Umbach50–Reference Zhang, Chen and Qiu52). The first, by Kamel and colleagues, reported a negative association between total fat intake and PD risk(Reference Kamel, Goldman and Umbach50). Although they demonstrated the same for dietary MUFA, SFA, PUFA, α-linolenic acid and linoleic acid, associations were greatest for α-linolenic acid and weakest for SFA(Reference Kamel, Goldman and Umbach50). In contrast, Wang and colleagues found no association between total fat intake and PD risk, however, they did report an inverse relationship between PD risk and intake of total PUFA, n-3 PUFA, n-6 PUFA and linoleic acid(Reference Wang, Lin and Wu51). The third study by Zhang and colleagues had a wider scope whereby they examined the relationship between fish and PUFA intake and PD risk in patients with mild-to-severe cognitive impairment(Reference Zhang, Chen and Qiu52). They reported that greater PUFA intake decreased PD risk, although not through the intake of the PUFAs DHA, EPA and α-linolenic acid(Reference Zhang, Chen and Qiu52). In summary, evidence about the intake of total fat and individual fatty acids is inconclusive and further research is needed in this area.
Food and food groups
Epidemiological evidence for the neuroprotective effects of caffeine-containing food and beverages, particularly from coffee and tea, emerged in the early 2000s(Reference Ross, Abbott and Petrovitch53–Reference Hu, Bidel and Jousilahti61). Meta-analyses which included several of these cohorts in addition to smaller case–control studies(Reference Tan, Chua and Fook-Chong62–Reference Tanaka, Miyake and Fukushima69) have consistently found an inverse association between caffeine intake and PD risk(Reference Liu, Guo and Park70–Reference Hong, Chan and Bai72). Hong and colleagues also included PD cohorts and suggested that consuming caffeine may slow the rate of disease progression(Reference Hong, Chan and Bai72–Reference Scott, Macleod and Counsell75). Recent case–control studies not included in these meta-analyses further support this inverse relationship between caffeine intake and PD risk(Reference Bakshi, Macklin and Hung76). The novel treatment istradefylline, recently approved for the treatment in PD, acts at the same adenosine A2A receptor as caffeine, which further supports this position(Reference Torti, Vacca and Stocchi77).
Cohort studies report positive associations between dairy intake and the risk of developing PD(Reference Kyrozis, Ghika and Stathopoulos46,Reference Sääksjärvi, Knekt and Lundqvist58,Reference Hughes, Gao and Kim78–Reference Park, Ross and Petrovitch81) . In the Health Professionals Follow-Up Study and Nurses' Health Study cohorts, dairy intake was reported to be positively associated with PD; men consuming ≥2⋅9 servings/d had an 80 % increased risk compared to those consuming <1 serving/d(Reference Chen, Zhang and Hernán80). More recent data from these cohorts found total dairy intake was not significantly associated with PD, while low-fat dairy intake was(Reference Hughes, Gao and Kim78), perhaps suggesting that the fat component of dairy foods may not increase PD risk(Reference Hughes, Gao and Kim78). The American Cancer Society's Cancer Prevention Study II found a positive association between dairy consumption in both sexes and PD risk; those in the top quintile had a risk ratio 1⋅6 greater compared to the lowest(Reference Chen, O'Reilly and McCullough79). The Honolulu Heart Program which followed up men over 30 years, found those consuming the most milk (>450 ml/d) had a 2⋅3-fold excess of PD v. non-milk drinkers, and no association was found for calcium from dairy or non-dairy sources(Reference Park, Ross and Petrovitch81). In prospective cohorts in Greece and Finland, associations were also observed between milk consumption and the increased risk of PD(Reference Kyrozis, Ghika and Stathopoulos46,Reference Sääksjärvi, Knekt and Lundqvist58) . In the Finnish cohort, a positive association was reported between reduced-fat dairy products and PD risk(Reference Sääksjärvi, Knekt and Lundqvist58). A meta-analysis including several of these studies(Reference Kyrozis, Ghika and Stathopoulos46,Reference Sääksjärvi, Knekt and Lundqvist58,Reference Chen, O'Reilly and McCullough79–Reference Park, Ross and Petrovitch81) , estimated the absolute risk differences were two–four PD cases per 100 000 person-years for every 200 g/d increment in milk intake, and one–three PD cases per 100 000 person-years for every 10 g/d increment in cheese intake(Reference Jiang, Ju and Jiang82). In turn, this was included in two umbrella reviews which considered it as Class III evidence(Reference Bellou, Belbasis and Tzoulaki16) and low by AMSTAR and GRADE scores(Reference Zhang, Chen and Xu83). Despite the positive associations between dairy intake and PD risk, there is currently not sufficient evidence to advise against dairy consumption at a population level.
Dietary patterns
Studies which focus on single nutrients and foods risk missing the synergistic effects at a whole diet level. Dietary patterns with well-established relationships to cardiovascular and metabolic health(Reference Chrysohoou, Panagiotakos and Pitsavos84–Reference Sofi, Abbate and Gensini86) have been investigated for their potential neuroprotective properties(Reference Sääksjärvi, Knekt and Lundqvist58,Reference Gu, Brickman and Stern87–Reference Anastasiou, Yannakoulia and Kosmidis89) . Although many studies used the healthy eating index and alternate healthy eating index to explore relationships between diet and neurological outcomes, in recent years indices based on the ‘Mediterranean diet’ (MeDi) have generated greatest research interest(Reference Gardener and Caunca90). Data from two US cohorts found that those in the highest alternate healthy eating index and alternate Mediterranean diet score quintiles were 30 and 25 %, respectively, less likely to develop PD v. the lowest(Reference Gao, Chen and Fung88); similar relationships were observed at later follow-up(Reference Molsberry, Bjornevik and Hughes91). However, a Finnish cohort using a modified alternate healthy eating index found no such relationship(Reference Sääksjärvi, Knekt and Lundqvist58). In a Greek population, MeDi adherence was associated with better cognitive performance and lower dementia rates(Reference Anastasiou, Yannakoulia and Kosmidis89), and subsequently it was reported that adherence was associated with fewer prodromal PD symptoms(Reference Maraki, Yannakoulia and Stamelou92). In a cohort of over 47 000 Swedish women, greater adherence to a MeDi at middle age was associated with a lower risk for PD(Reference Yin, Löf and Pedersen93). Studies have examined a combined Mediterranean-Dietary Approaches to Stop Hypertension Diet Intervention for Neurodegenerative Delay(Reference Metcalfe-Roach, Yu and Golz94,Reference Agarwal, Wang and Buchman95) : Agarwal and colleagues found that it appeared to reduce PD risk and slow disease progression whereas neither the MeDi nor Dietary Approaches to Stop Hypertension diets alone achieved this(Reference Agarwal, Wang and Buchman95). Moreover, in a smaller study, Metcalfe-Roach and colleagues found similar findings but in women only, whereas a Greek MeDi was protective in both sexes. There has been speculation that other diets such as the ketogenic, vegetarian and vegan can reduce PD risk although the evidence is limited(Reference Włodarek96). Interest in plant-based diets and PD stems from the protective attributes of the MeDi or other diets rich in fruit and vegetables(Reference Okubo, Miyake and Sasaki97), and the apparent lower prevalence of PD outside of North America and Europe, where animal-derived foods form a smaller proportion of dietary intake.
Whole body metabolism in Parkinson's disease
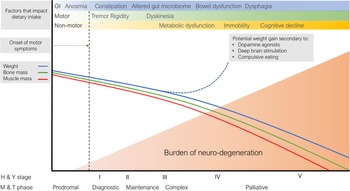
Impaired nutritional status is a common occurrence in patients once they have established features of PD and weight loss may occur with PD progression, holding implications for treatment, quality of life and mortality. Numerous factors occur that may contribute to this process across the course of the condition, visually conceptualised in Fig. 2.

Fig. 2. Putative representation of the dynamic and interacting factors that impact nutritional status in Parkinson's disease. GI, gastrointestinal; M & T, MacMahon and Thomas(Reference MacMahon and Thomas8); H & Y, Hoehn and Yahr(Reference Hoehn and Yahr6).
Body weight
One way in which nutritional dysfunction can be observed in PD is the variation in body weight that is observed throughout the lifespan of the disease. Conflicting results have been reported in epidemiological studies likely due to the considerable heterogeneity between different populations and varying stages of the condition when studied. The investigation of weight in populations with PD poses a multitude of difficulties with confounding occurring at a population level with various levels of obesity in the country in which the study was undertaken, coupled with confounders from comorbidities such as diabetes, as well as the drug treatment diet regimens. Furthermore, the possible existence of specific disease phenotypes in which weight loss may feature differentially may further complicate the picture(Reference Sharma and Turton98). Weight loss in PD may predispose patients not only to an increased risk of malnutrition, but has been postulated to lead to the worsening of symptoms(Reference Sharma and Turton98).
Changes in body weight may occur as a prodromal feature, before the typical symptoms of PD can be observed in an individual, however there are conflicting results. In a prospective study, Chen and colleagues demonstrated significant weight loss prior to diagnosis in over four hundred patients with PD(Reference Chen, Zhang and Hernán99). They suggested that the average weight of patients with PD was stable until shortly before diagnosis and then declined. However, in a case–control study, Ragonese and colleagues reported that no change in BMI occurred during the period preceding disease onset(Reference Ragonese, D'Amelio and Callari100). Both studies used different methods and assessed two geographically disparate populations some decades apart. When symptomatic individuals with PD have been studied, the literature is also inconclusive. A low BMI in symptomatic individuals with PD, relative to healthy controls, has been demonstrated in a meta-analysis of twelve studies and in over eight hundred patients(Reference van der Marck, Dicke and Uc101). Weight variation over the disease course is of increasing interest and is relevant clinically as the therapeutic window of the drug treatments diminishes over time and therefore dosing needs to be reviewed in light of weight changes. Yong and colleagues found progressive weight loss in PD in a 3-year longitudinal study(Reference Yong, Tan and Ng102), whereas a further cross-sectional study of n = 125 adults with a median PD duration of 6 years reported unintentional weight loss in 38 and 50 % of men and women, respectively(Reference Sheard, Ash and Mellick103). A study of a smaller cohort also suggested that BMI is low early in PD; however, a retrospective chart review was used to collect height and weight data in that instance(Reference Cheshire and Wszolek104). Other studies have shown no evidence of low weight or BMI in PD. Barichella and colleagues assessed BMI in n = 364 Italian men and women with a mean duration of PD of 10⋅6 years and found 65 % of the cohort were overweight(Reference Barichella, Marczewska and Vairo105). Similarly, when compared with controls in a cross-sectional study, overweight and obesity were more common among n = 177 Mexican patients with PD(Reference Morales-Briceño, Cervantes-Arriaga and Rodríguez-Violante106). Where it has been demonstrated, a low BMI in PD has been shown to increase the risk of cognitive decline(Reference Kim, Oh and Lee107), and is a risk factor for mortality, particularly in males(Reference Park, Oeda and Kohsaka108), and has also been correlated with decreased olfaction(Reference Sharma and Turton98). Early weight loss, occurring within the first year, has been associated with excess mortality(Reference Cumming, Macleod and Myint109).
Energy expenditure
Changes in energy expenditure have been investigated in PD. Increased energy expenditure has been demonstrated in a number of studies(Reference Ma, Xiong and Shen110) associated, in particular, with rigidity and the medication ‘off’ state which are periods of time in which symptoms significantly worsen, usually due to wearing off of dopaminergic medication(Reference Ross, Abbott and Petrovitch111,Reference Abbott, Ross and Petrovitch112) . Further studies have shown either no evidence of increased resting energy expenditure or even a lower energy expenditure associated with the decreased level of physical activity that occurs due to disability in PD(Reference Barichella, Cereda and Faierman113,Reference Toth, Fishman and Poehlman114) . Investigating this association is likely confounded by the dynamic nature of ‘off’ and ‘on’ medication states and the influence of varying medication states that can change throughout the course of a day.
The symptom of tremor, which predominantly occurs at rest, is a potential cause of an increase in energy expenditure. This association has seldom been investigated aside from extrapolation based on the observation of weight gain following deep brain stimulation (DBS) surgery(Reference Tuite, Maxwell and Ikramuddin115). In contrast, in studies where DBS has been carried out for the unlinked condition of essential tremor, weight gain has not been shown to be significant, disputing the link to increased energy expenditure due to excess movement from tremor(Reference Strowd, Cartwright and Passmore116). Excess energy can also be spent with the excessive movement associated with dyskinaesia, a fidgeting-like motion occurring due to a paradoxical surfeit of dopamine. This is common in association with levodopa therapy, with the risk worsening throughout the disease course, and is associated with a decreased body weight(Reference Yong, Tan and Ng102). The link of DBS with weight gain may also be a function of this negative effect of dyskinaesia on weight, as one of the primary indications for undertaking DBS is to improve the nature of dyskinaesia, particularly by reducing required levodopa dosing. Additionally, a central metabolic effect may be observed given the close proximity of one DBS target, the subthalamic nucleus, to the hypothalamus(Reference Steinhardt, Münte and Schmid117).
Energy intake
Alterations in energy intake have also been found in PD. Changes in the quality of the diet may be driven by specific cognitive changes pertaining to food selection or as part of an impulse control disorder, the latter of which may be related to dopaminergic medication(Reference Kelly, Baig and Hu118). Compulsive eating has been documented in PD, with one case–control study suggesting that 25 % of patients with PD consumed excessive energy compared with 12 % of healthy controls(Reference de Chazeron, Durif and Lambert119). This can manifest as binge-eating and correlates with the presence of anxiety more strongly than medication type or dose(Reference de Chazeron, Durif and Chereau-Boudet120). Dietary intake in PD may be subtly affected by more than simply over-eating, with several studies suggesting a preference for sweet foods including chocolate, cakes and ice cream, even relative to household controls(Reference Lorefält, Granérus and Unosson121–Reference Wolz, Kaminsky and Löhle124). Higher carbohydrate intake, associated with lower protein, folate, magnesium and phosphorus intake has also been observed(Reference Ådén, Carlsson and Poortvliet125). Other studies have examined the intake of food groups and low intake of fruit, vegetables and meat has been observed(Reference Lorefält, Granérus and Unosson121). The dopaminergic system is highly implicated in reward and motivation, and dysfunction likely contributes to the pattern of abnormal eating observed in PD, as suggested empirically by the association of dopamine agonist therapy with overeating. It has been suggested that overeating of rewarding foods that triggers a small dopamine ‘hit’ may be in response to the more generalised hypodopaminergic state that defines PD(Reference Ådén, Carlsson and Poortvliet125).
Metabolic homoeostasis
Evidence for dysfunction of metabolic homoeostasis has been found in PD, in particular pathology affecting the hypothalamus as demonstrated in post-mortem and functional imaging studies(Reference Ma, Xiong and Shen110). Decreased orexin is observed in the cerebrospinal fluid of individuals with PD(Reference Drouot, Moutereau and Lefaucheur126,Reference Chieffi, Carotenuto and Monda127) , although correlation with weight has not been undertaken. Decreased ghrelin is observed in the plasma of patients with PD who experience weight loss. This suggests a degree of neurohormonal dysregulation, with an opposite relationship (an increase) usually observed with weight loss in healthy subjects(Reference Chieffi, Carotenuto and Monda127,Reference Fiszer, Michałowska and Baranowska128) . Decreased dynamic control of ghrelin has been demonstrated at prodromal stages, prompting the question of whether this is an early symptomatic change or causative mechanism(Reference Unger, Möller and Mankel129). Although conflicting results for leptin levels have been published in recent years, a recent meta-analysis suggested that this remains normal(Reference Rahnemayan, Mirghafourvand and Fathalizadeh130). Evidence exists for a role of mitochondrial dysfunction in the aetiology of PD(Reference Park, Davis and Sue131), suggesting that an intrinsic dysfunction of energy handling may exist in affected individuals, with neuronal cells particularly sensitive to abnormalities of cellular respiration.
Malnutrition, muscle and bone
Malnutrition is common in older adults and is prevalent in PD. In a systematic review, Sheard and colleagues assessed the prevalence in over 1100 patients with PD in eleven studies. The mean age of the PD samples ranged from 54 to 75 years and the duration of the disease ranged from 5 to 13 years. Irrespective of whether the patient was hospitalised, measured in a PD clinic or living in the community, the prevalence of malnutrition in PD ranged from 0 to –24 % and the risk of malnutrition from 3 to 60 %(Reference Sheard, Ash and Silburn132). Other studies of either hospitalised patients(Reference Yang, Zhan and Zhang133) or those attending an outpatient clinic(Reference Paul, Singh and Paul134) support this finding, although different methods were used to measure malnutrition across the studies. In addition to being at an increased risk of malnutrition because of the symptoms of PD, the treatment used to manage PD may also increase malnutrition risk(Reference Sheard, Ash and Silburn132).
Malnutrition is a known risk factor for sarcopaenia(Reference Beaudart, Sanchez-Rodriguez and Locquet135–Reference Gómez-Gómez and Zapico137), the age-related loss of muscle and strength (Reference Morley, Baumgartner and Roubenoff138,Reference Cruz-Jentoft, Baeyens and Bauer139) , which combined with low serum 25(OH)D in PD may negatively impact muscle strength and balance, increasing falls risk(Reference Hiller, Murchison and Lobb35). Determining the prevalence of sarcopaenia in PD is complicated by a lack of consensus and a multitude of clinical definitions in widespread use. These include The European Working Group on Sarcopenia in Older Persons (EWGSOP-1 and EWGSOP-2)(Reference Cruz-Jentoft, Baeyens and Bauer139,Reference Cruz-Jentoft, Bahat and Bauer140) , Foundation of the National Institutes of Health(Reference Studenski, Peters and Alley141) and International Working Group on Sarcopenia(Reference Fielding, Vellas and Evans142) definitions. Although the European Society for Parenteral and Enteral Nutrition guideline for clinical nutrition in neurology suggests that sarcopaenia risk in PD is low(Reference Burgos, Bretón and Cereda143) they cite only a single study that used a clinical definition of sarcopaenia. This study of Italians with parkinsonian syndromes (n = 364) reported a prevalence of 7 % using the EWGSOP-1 definition(Reference Barichella, Pinelli and Iorio144). Several more recent studies have reported higher rates. A Turkish case–control study (n = 155) used EWGSOP-1 and reported a prevalence of 50 % in PD and 31 % in controls(Reference Ozer, Akın and Gultekin145), whereas a Brazilian study reported a prevalence of 22 % in PD using EWGSOP-2 compared to 55 % with EWGSOP-1(Reference da Luz, Bezerra and Asano146). A recent Malaysian study reported a prevalence of 26 % in PD using EWGSOP-2 and 4 % in controls(Reference Tan, Lim and Yong147), greater than previous Malaysian rates based on The Asian Working Group on Sarcopenia criteria(Reference Chen, Liu and Woo148). Vetrano and colleagues compared the criteria and found the prevalence of sarcopaenia in PD varied between 29 and 41 % in men and 18 and 33 % in women, with poor agreement between EWGSOP-1, Foundation of the National Institutes of Health and IGWS(Reference Vetrano, Pisciotta and Laudisio149).
Although in a young healthy individual, a fall from standing height would not likely result in a fracture, in PD, reduced bone mineral density(Reference Torsney, Noyce and Doherty150–Reference Tan, Wang and Zhou152) and a higher frequency of falls increase fracture risk(Reference Zhang, Zhang and Mao43,Reference Hely, Reid and Adena153–Reference Lee, Choi and Shin159) . Studies suggest that in PD, osteoporosis and fracture are twice as likely(Reference Torsney, Noyce and Doherty150,Reference Kalilani, Asgharnejad and Palokangas160,Reference Sleeman, Che and Counsell161) , and in women, PD is the strongest single contributor to fracture risk(Reference Dennison, Compston and Flahive162). Patients with PD also appear to have a greater prevalence of hip fracture(Reference Coomber, Alshameeri and Masia163–Reference Mühlenfeld, Söhling and Marzi165), which is associated with increased morbidity and mortality(Reference Walker, Chaplin and Hancock166,Reference Crego-Vita, Sanchez-Perez and Gomez-Rico167) . Of particular relevance to bone health, a number of case–control studies have suggested serum 25(OH)D is reduced in PD(Reference Evatt, Delong and Khazai33,Reference Ding, Dhima and Lockhart34,Reference Zhang, Zhang and Mao43,Reference Abou-Raya, Helmii and Abou-Raya168–Reference Peterson, Mancini and Horak170) , and those with lower 25(OH)D have a greater frequency of falls(Reference Zhang, Zhang and Mao43). Given the vital role that nutrition plays in musculoskeletal function, further research would be valuable to specifically determine the benefit of dietary interventions on bone health and fracture risk.
The effects of Parkinson's disease symptoms and treatment on nutritional status
Gastrointestinal symptoms
Anosmia and dysgeusia
Anosmia is a frequently reported symptom in PD, often occurring many years before the onset of motor symptoms and affecting up to 90 % of patients(Reference Tarakad and Jankovic171). Ageusia is less commonly reported, however taste is heavily dependent on the sense of smell and the two interlinked may affect up to 27 % of patients with PD(Reference Shah, Deeb and Fernando172). Other symptoms such as xerostomia may contribute to taste abnormalities. A decreased sense of smell and taste is known to negatively influence nutritional status(Reference Andersson and Sidenvall173). On examination of the relationship between anosmia, dysgeusia and body weight, it was found that olfactory and gustatory deficits may negatively influence weight(Reference Masala, Loy and Piras174). In a prospective study of patients with PD, Sharma and Turton found that 39 % of patients were characterised as ‘weight losers’ and 60 % as ‘non-weight losers’, with ‘weight losers’ more likely to be older, female and have more severe olfactory impairment(Reference Sharma and Turton98). Since smell is an important aspect of food appeal, it is possible that altered olfaction can negatively impact dietary intake that may be important in the pathophysiology of PD(Reference Ådén, Carlsson and Poortvliet125). Ådén and colleagues found associations between a lower intake of nutrients such as protein and olfactory function in patients newly diagnosed with PD compared to a control group(Reference Ådén, Carlsson and Poortvliet125). Roos and colleagues suggested that hyposmia, and not hypogeusia, may contribute to weight loss in PD and hence increase the risk of malnutrition. This cross-sectional study found a significant correlation between olfactory function and BMI, but not between gustatory function and BMI(Reference Roos, Oranje and Freriksen175).
Dysphagia and the oral cavity
Dysphagia is common in PD, having an estimated symptomatic prevalence of 35 % based on a meta-analysis of ten studies. The prevalence is further increased to 82 % when considering objective measures of swallowing function(Reference Kalf, de Swart and Bloem176), suggesting that asymptomatic dysphagia is widespread and occurs silently. Symptoms arise due to the disruption of swallowing at all stages of the process including the oral stage (reduced oral bolus control), pharyngeal phase (impaired coordination of pharyngeal structures) and the oeosophageal stage (impaired sphincter relaxation)(Reference Simons177). Hypersalivation or conversely xerostomia that can be linked to levodopa therapy are other factors that may impair the swallowing of food(Reference Umemoto, Fujioka and Iwasa178). Patients with PD also suffer from worse dentition than similarly aged individuals, further impairing their ability to eat, with tooth decay in turn contributed to by xerostomia(Reference Hanaoka and Kashihara179). Orofacial pain phenomena such as burning mouth syndrome occur at an increased prevalence in PD, causing potential distress with eating(Reference Coon and Laughlin180). Facial and lip tremors may also occur, affecting confidence with eating(Reference Ou, Wei and Hou181). Each of these factors can individually or collectively impair dietary intake and have a negative impact on nutritional status. Moreover, difficulties ingesting food may impair the intake of specific micronutrients such as vitamin D and calcium, subsequently increasing the risk of osteoporosis and fracture risk. Swallowing issues can also affect quality of life(Reference Carneiro, das Graças Wanderley de Sales Coriolano and Belo182) which may be directly through embarrassment in social situations, or due to fear induced by choking episodes, as well as the impact that dysphagia can have on dietary intake. The presence of dysphagia is a risk factor for adverse outcomes such as pneumonia through silent aspiration, a primary cause of death associated with PD(Reference Pennington, Snell and Lee183).
Changes to the gut microbiome
Small bowel gastrointestinal overgrowth has been commonly observed in PD, affecting an estimated 46 % of patients and is characterised by an overgrowth of bacterial colonies beyond normally observed limits or the growth of abnormal types(Reference Li, Feng and Jiang184). Diagnosis relies on the use of a hydrogen breath test to infer overgrowth, as used in studies defining prevalence in PD, although the gold standard is the physical culture of jejunal aspirate. The gut microbiome in PD is well documented to be abnormal relative to healthy controls and the overabundance of particular species are associated with the risk of GI symptoms in PD such as constipation(Reference Boulos, Yaghi and El Hayeck185). Vitamin B12 deficiency can result from the consumption of this vitamin by anaerobic micro-organisms(Reference Bures, Cyrany and Kohoutova186) and bacteria can disrupt bile acid which adversely affects the absorption of fat and fat-soluble vitamins(Reference Bures, Cyrany and Kohoutova186). Hasuike and colleagues hypothesised that bacterial overgrowth has various effects via bile acid metabolism in PD(Reference Hasuike, Endo and Koroyasu187). It has been suggested that serum bilirubin increases as bilirubin metabolism declines with decreases in the intestinal bacteria(Reference Hasuike, Endo and Koroyasu187). Simultaneously, bile acid is degraded due to increased intestinal bacteria, and lipid absorption decreases leading to low serum TAG levels and loss of body mass(Reference Hasuike, Endo and Koroyasu187). Similarly, there is decreased absorption of vitamin D aligned with a decrease in bile acid, a risk factor for osteoporosis and fractures. It is clear that consideration needs to be given to the hypothesis that some of the non-motor manifestations accompanying PD are caused by intestinal dysbiosis(Reference Hasuike, Endo and Koroyasu187). Helicobacter pylori (H. pylori) infection has been linked to PD, with higher rates of infection observed PD(Reference Dardiotis, Tsouris and Mentis188). The benefit of eradication therapy has been addressed and is linked to improved outcomes such as UPDRS score and ‘on’ time(Reference Bai and Li189), suggesting that untreated infection may impair levodopa absorption.
Constipation
The most commonly recognised GI symptom in PD is constipation, referring to both difficulty defecating and a decreased frequency of stool, and affects between 20 and 81 % of patients(Reference Sakakibara190). Constipation can be observed as a prodromal symptom predating the onset of defining motor symptoms(Reference Picillo, Palladino and Erro191), and is linked to worse outcomes that include earlier onset of dementia(Reference Camacho, Macleod and Maple-Grødem192). Symptoms arise from the degeneration of brain structures involved in controlling defecatory storage and expulsion, as well as the nerves of the myenteric plexus within the bowel that control motility(Reference Ohlsson and Englund193). A similar effect on motility can also be observed in the upper GI tract, causing delayed gastric emptying(Reference Yu, Ramsey and Norton194) and both can adversely affect quality of life. Bowel motility issues also affect dopaminergic medication absorption(Reference Doi, Sakakibara and Sato195), worsening motor control and cause further impact on quality of life. Constipation is a risk factor for malnutrition(Reference Fávaro-Moreira, Krausch-Hofmann and Matthys196). In a sample of community-dwelling patients with PD, nutritional status was measured using two validated tools: the Subjective Global Assessment and the Patient-Generated Subjective Global Assessment(197). Forty three percent of the cohort reported previous unintentional weight loss following diagnosis, and 15 % were moderately malnourished wherein constipation was one of the symptoms reported to adversely affect dietary intake(Reference Sheard, Ash and Mellick103). Sheard and colleagues assessed nutritional status using the Patient-Generated Subjective Global Assessment in patients with PD awaiting DBS surgery, and of the nutrition impact symptoms listed, 58 % recorded that constipation adversely affected their dietary intake over the previous 2 weeks(Reference Sheard, Ash and Silburn198). In a cross-sectional study of PD in China, constipation was considered to be one of the two most important predictors of nutritional impairment(Reference Wang, Wan and Cheng199). Delayed gastric emptying and the resultant nausea, bloating and early satiety(Reference Yu, Ramsey and Norton194) can adversely affect appetite and in turn dietary intake, and increase the risk of malnutrition in this patient group.
The effect of non-gastrointestinal symptoms on nutritional status
Difficulty with eating
Patients with PD experience a motor disorder that affects manual dexterity and control, therefore leading to problems with the physical act of eating. Difficulty manipulating food on the plate and transporting it to the mouth accurately is observed with the risk of spillage due to tremor(Reference Athlin, Norberg and Axelsson200). Increased upper limb tremor has been associated with a lower energy intake, as has the observation of fewer spoonfuls being taken during a meal by individuals with advanced stage disease(Reference Fagerberg, Klingelhoefer and Bottai201) and represents the direct effect of the cardinal motor features of PD on the risk of malnutrition.
Cognition
In PD, cognitive impairment is a common symptom later in the disease course. Specific decline in frontal lobe function has been shown to correlate with the BMI, fitting with the potential sweet food preference that can be observed in frontal executive dysfunction(Reference De Lucia, Peluso and Esposito202). Cognitive impairment as it progresses becomes a risk factor for becoming dependent on others for food intake(Reference Jung, De Gagne and Lee203). Individuals are less likely to ask for food and less likely to be able to physically prepare or access food at will due to motor disability in PD, especially in an environment that is unfamiliar such as physical care settings. Overall, the prevalence of malnutrition in care homes is high, estimated at approximately 15 % worldwide(Reference Kaiser, Bauer and Rämsch204). This may be impacted by staffing constraints commonly encountered in such environments, and a lack of social interaction may also remove some aspects of reward associated with eating(Reference Liu, Jao and Williams205). Depression is also a common occurrence in PD and may also negatively influence volition to eat with a demonstrated association of weight loss(Reference Reijnders, Ehrt and Weber206,Reference Kim, Chung and Yoo207) .
The effect of treatment on nutritional status
Dopaminergic-based treatments for PD primarily target motor symptoms, but are associated with recognised side-effects (Table 1). Dopaminergic medication, including levodopa, dopamine agonists and catechol-O-methyltransferase inhibitors commonly cause nausea and vomiting which is usually transient during an initial period of acclimatisation, but may adversely affect dietary intake in the short term. Dopamine agonist medications are associated with weight gain in the long term(Reference Wills, Li and Pérez208,Reference Artaud, Lee and Mangone209) , potentially due to an increased incidence of compulsive eating associated with impulse control disorder. Levodopa use has conversely been associated with a decrease in body weight, although historically dopamine agonists have been used in earlier disease and may therefore simply be a function of disease staging. Weight loss is also observed with levodopa intestinal gel-infusions; however, this is also a treatment reserved for late-stage disease(Reference Fabbri, Zibetti and Beccaria210). Dyskinaesia is another common phenomena related to levodopa therapy and is associated with increased weight loss(Reference Yong, Tan and Ng102).
Table 1. Common medications and their effects on nutritional status

MAO, monoamine oxidase type B; NMDA, N-methyl-d-aspartate.
Adapted from BDA Best Practice guidance for dietitians on the nutritional management of Parkinson's, 2021(219).
Medications can also influence the handling of nutrients important for metabolism. As such, raised homocysteine levels are observed in those patients with oral levodopa dosing and decreased vitamins B12, B6 and folate levels associated with intestinal gel use(Reference Muhlack, Kinkel and Herrman211,Reference Taher, Naranian and Poon212) . As described previously, DBS has been found to be consistently associated with weight gain. This may result from an improvement in dyskinaesia, a primary indication for this therapy, thus reducing overall energy expenditure(Reference Balestrino, Baroncini and Fichera213).
Nutritional status is known to affect the response to PD treatments, likely by affecting pharmacokinetic handling. Individuals with lower body weight are at an increased risk of peak-dose dyskinaesia on levodopa, with a greater area under the curve exposure and longer elimination observed, necessitating the consideration of weight and anticipation of ongoing nutritional care when dosing(Reference Arabia, Zappia and Bosco214–Reference Zappia, Crescibene and Arabia216). When normalised for body weight and adjusted for age, sex and disease severity, levodopa has been associated with the impaired nutritional status in a dose-dependent manner(Reference Adams, Boschmann and Lobsien217). In addition, a cumulative dose of levodopa has been reported to be associated with micronutrient deficiency through changes in homocysteine, vitamin B6 and B12 levels(Reference Ceravolo, Cossu and Bandettini di Poggio218). Other common medications used in the treatment of PD and the effects that they may have on nutritional status are described in Table 1(219).
Conclusions and future research
We have presented an overview of nutrition in PD in this review and have covered three domains: dietary intake and the development of PD; whole body metabolism in PD, including energy balance and musculoskeletal health and the effects of PD symptoms and treatment on nutritional status. We have highlighted areas where future research on the effect of PD on nutritional status is particularly relevant and important. Determining modifiable risk factors for PD with further research specifically on Mediterranean-style diets in particular, the Mediterranean-Dietary Approaches to Stop Hypertension Diet Intervention for Neurodegenerative Delay diet, could be vital in enhancing our knowledge of the pathophysiology of PD and therefore the development of novel treatments for the condition. Additionally, there is evidence that patients with PD are at risk of malnutrition and a better understanding of the mechanisms behind this is important in developing holistic care to optimise outcomes and quality of life.
Little is known about appetite in PD aside from limited studies that explore a tendency towards certain foods. Additionally, the burden of disability that can affect dexterity, cognitive impairment and the motor impairments that negatively impact the physical act of eating have not yet been fully determined. Tackling this need would enable techniques to be developed to assist with eating and thus improve nutritional status in this patient group. We have highlighted the studies that have sought to characterise patients' weight in the years before the onset of symptoms. Further research will allow us to understand the role of weight change in the pathophysiology of PD, addressing the question of whether weight change is integral to the underlying pathology or merely an additional early symptom.
Much has been discussed about the link between PD and the GI tract, archetyped by the Braak hypothesis(Reference Braak, Del Tredici and Rüb11) although currently only circumstantial evidence exists. The role played by empirically observed phenomena such as small bowel gastrointestinal overgrowth is not yet fully understood, in particular the extent to which this is important in the prodromal phase, nor is the impact of H. pylori infection, where the impact on dyskinaesia is not yet clear. Adequately powered and well-designed randomised controlled trials are required to assess these links.
A better understanding of the role that nutritional factors play in aetiology will further understand and may, in due course, contribute to developing interventional strategies to augment care and improve symptoms for patients living with this complex and multifactorial neurodegenerative disease.
Acknowledgements
We gratefully acknowledge the input of Stephanie Drake who illustrated Fig. 1 on behalf of the authors.
Financial Support
All authors receive salary support from the Gatsby Foundation.
Conflict of Interest
None.
Authorship
The authors were solely responsible for all aspects of preparation of the present review.