Introduction
Pregnant women undergo significant physiologic changes throughout pregnancy and the peripartum period. These changes impact every organ system, allowing the mother to adapt to the demands of the developing fetus.
Specialists in maternal-fetal anesthesia often care for women with mild pre-existing disease or pregnancy-related pathology, both of which can have significant impact on maternal physiology. While these pathophysiological changes often require alterations in medical management, this chapter focuses on the normal physiologic changes that occur in a healthy woman during pregnancy. A clear understanding of the normal alterations to physiology during pregnancy provides the foundation for managing the full spectrum of patients that can present to the maternal-fetal anesthesia specialist.
Cardiovascular System
Anatomic Changes
During pregnancy, the cardiovascular system must physically adapt to the changes occurring in the body. The heart muscle and vasculature respond to increases in intravascular volume and metabolic demand. As the uterus grows and the diaphragm becomes elevated, the heart’s position in the chest changes as well.
Generally, the left ventricle (LV) size increases along with the increased cardiac work of pregnancy, preserving the myocardial oxygen supply–demand relationship. In the setting of increased preload and afterload, this eccentric hypertrophy is thought to be an adaptation to minimize wall stress, and appears similar to the cardiac response to exercise.Reference Robson, Dunlop, Moore and Hunter1–Reference Melchiorre, Sharma and Thilaganathan4 Growth of the left ventricle begins as early as the first trimester, reaching a 15–25% increase in ventricular wall thickness and a 50% increase in overall LV mass at term.Reference Robson, Dunlop, Moore and Hunter1–Reference Simmons, Gillin and Jeremy3, Reference Vered, Mark Poler, Gibson, Wlody and Pérez5, Reference Gilson, Samaan, Crawford, Quails and Curet6
These cardiovascular changes are notable both on physical exam and diagnostic studies, particularly as the pregnancy nears term. Electrocardiogram (ECG) changes include shortening of the PR and QT intervals, QRS axis variability, depressed ST segments, and isoelectric low-voltage T waves in left-sided leads.Reference Carruth, Mirvis, Brogan and Wenger7, Reference Oram and Holt8 Echocardiography reveals an increase in valve annulus diameters associated with evidence of tricuspid and pulmonic regurgitation in up to 94%, and mitral regurgitation in up to 27% of healthy pregnant women at term.Reference Campos, Andrade and Bocanegra9
Hemodynamic Changes
Hemodynamic changes during normal pregnancy are relatively predictable. An overview of these changes is shown in Table 1.1. Generally, cardiac output increases, blood pressure remains relatively stable, and total vascular resistance decreases.Reference Melchiorre, Sharma and Thilaganathan4
Table 1.1 Summary of cardiovascular hemodynamic changes during pregnancy
| Parameter | Change in pregnancy |
|---|---|
| Heart rate | ⇑ |
| Stroke volume | ⇑ |
| Cardiac output | ⇑⇑ |
| Ejection fraction | ⇑ |
| Systemic vascular resistance | ⇓ |
| Systolic blood pressure | – |
| Diastolic blood pressure | ⇓ |
| Mean arterial pressure | ⇓ – |
| Central venous pressure | – |
| Pulmonary vascular resistance | ⇓ |
| Pulmonary artery pressure | – |
Cardiac Output, Heart Rate, and Stroke Volume
Cardiac output is the quantity of blood pumped by the heart each minute and is the product of heart rate and stroke volume. While the magnitude and time course of changes in heart rate and stroke volume throughout pregnancy are controversial because of variations in measurement, both increase from baseline values during pregnancy. The increase in heart rate begins early in the first trimester and peaks in the third trimester at levels around 15–25% higher than baseline.Reference Robson, Dunlop, Moore and Hunter1, Reference Melchiorre, Sharma and Thilaganathan4, Reference Duvekot, Cheriex, Pieters, Menheere and Peeters10–Reference Atkins, Watt, Milan, Davies and Crawford12 Stroke volume reaches values of 20–30% above baseline by the second trimester.Reference Melchiorre, Sharma and Thilaganathan4, Reference Robson, Hunter, Boys and Dunlop13–Reference Pöpping, Elia, Marret, Wenk and Tramr16 Because the left ventricular end-diastolic volume increases but the end-systolic volume remains unchanged, the ejection fraction is increased relative to nonpregnant levels.Reference Robson, Hunter, Boys and Dunlop13
As heart rate and stroke volume increase, cardiac output rises, with higher values noted as early as 5 weeks’ gestation.Reference Robson, Hunter, Boys and Dunlop13 By the end of the first trimester, cardiac output reaches up to 30–40% above baseline. Cardiac output continues to increase through the second trimester, reaching up to 50% higher than pre-pregnancy values and remains stable during the third trimester in nonlaboring women.Reference Robson, Hunter, Boys and Dunlop13, Reference Laird-Meeter, van de Ley, Bom, Wladimiroff and Roelandt17–Reference Clark, Cotton and Lee19 This rise in cardiac output serves to increase uterine blood flow from 50 mL/min in nonpregnant women to 700–900 mL/min (over 10% of cardiac output) at term.Reference Assali, Douglass, Baird, Nicholson and Suyemoto20, Reference Thaler, Manor and Itskovitz21 Blood flow also increases to the kidneys, skin, and breast tissue.Reference Katz and Sokal22–Reference O’Day24
Maintaining cardiac output for adequate uterine perfusion is critical for anesthesiologists when managing hemodynamics during an anesthetic; this is particularly important when neuraxial anesthesia leads to a sympathectomy. Phenylephrine has replaced ephedrine as the preferred first-line vasopressor in pregnant women; it is now commonly used to treat low blood pressure associated with neuraxial anesthesia.Reference Macarthur and Riley25 Following vasopressor administration, changes in cardiac output correlate with changes in heart rate, making heart rate a surrogate indicator of cardiac output in these patients.Reference Dyer, Reed and van Dyk26 That is, when treating decreased systemic vascular resistance, phenylephrine should be dosed with the goal of maintaining heart rate and avoiding reflex bradycardia, which could lead to a decrease in cardiac output.
Systemic Vascular Resistance and Blood Pressure
Systemic vascular resistance (SVR) falls during normal pregnancy, with a resultant increase in arterial compliance. This is an adaptive response to accommodate the significant elevation in intravascular volume that occurs in pregnancy.Reference Melchiorre, Sharma and Thilaganathan4 A nadir of 35% below baseline SVR occurs in the second trimester at around 20 weeks’ gestation. Subsequently the SVR begins to rise, returning to approximately 20% less than nonpregnant values at term.Reference Clark, Cotton and Lee19 The decrease in SVR during pregnancy is thought to be related to hormonally mediated vasodilation, as well as the development of the intervillous space, which serves as a low-resistance vascular bed.Reference Gaiser and Chestnut27
Blood pressure decreases concomitantly with changes in SVR, with a nadir around 28 weeks’ gestation. The decrease in diastolic blood pressure is more marked than that of systolic blood pressure, which does not change significantly during pregnancy.Reference O’Day24, Reference Gunderson, Chiang and Lewis28 Mean arterial pressure mirrors the changes in diastolic blood pressure, with a nadir in the second trimester followed by an increase to pre-pregnancy levels by term.Reference Simmons, Gillin and Jeremy3, Reference Mabie, DiSessa, Crocker, Sibai and Arheart29, 30
The gravid uterus can compress the inferior vena cava (IVC) and aorta, with the extent of the compression related to positioning and gestational age. Aortocaval compression may lead to hemodynamic disturbances with resultant uteroplacental hypoperfusion.Reference Ansari, Wallace, Clemetson, Mallikarjuneswara and Clemetson31 For this reason, left lateral tilt positioning is often recommended to achieve left uterine displacement, thereby reducing aortocaval compression.Reference Ansari, Wallace, Clemetson, Mallikarjuneswara and Clemetson31, Reference Kinsella32 Recent magnetic resonance imaging studies showed IVC but not aortic compression in term pregnant women; this caval compression was relieved by 30 (but not 15) degree lateral tilt positioning.Reference Abengochea, Morales-Roselló, Del Río-Vellosillo, Argente and Barberá33
Pulmonary Vascular Resistance
Pulmonary vascular resistance decreases during pregnancy.Reference Gaiser and Chestnut27 This decrease accommodates the increase in cardiac output without an elevation in pulmonary artery pressure as measured by pulmonary capillary wedge pressure.Reference O’Day24
Hemodynamic Changes During Labor
Classically, it has been taught that cardiac output increases by as much as 10–25% from pre-labor values in the first stage of labor and by 40% in the second stage of labor.Reference Robson, Hunter, Boys and Dunlop13, Reference Kjeldsen34 However, recent studies using minimally invasive continuous hemodynamic monitoring suggest that the progression of labor does not have a major effect on baseline hemodynamic values between contractions.Reference Kuhn, Falk and Langesæter35
During uterine contractions, 300–500 mL of blood is displaced from the intervillous space into the central circulation.Reference Hendricks36, Reference Adams and Alexander37 While studies reporting the absolute changes in cardiac output and stroke volume differ,Reference Gaiser and Chestnut27, Reference Kuhn, Falk and Langesæter35 it is agreed that the overall hemodynamic stress is substantially higher during the second stage of labor.Reference Kuhn, Falk and Langesæter35 Immediately after delivery, cardiac output has been reported to increase up to 75% more than pre-delivery measurements, but more recent studies with different monitoring techniques have questioned this value.Reference Kuhn, Falk and Langesæter35, Reference Adams and Alexander37, Reference Filippatos, Baltopoulos and Lazaris38 Cardiac output returns to pre-labor values at 24 hours and pre-pregnancy values by 12–24 weeks postpartum.Reference Kjeldsen34,Reference Hendricks36,Reference Adams and Alexander37,Reference Robson, Dunlop, Boys and Hunter39
Respiratory System
Pregnancy impacts both the anatomy and physiology of the respiratory system.
Anatomic Changes
As the rate of general anesthesia for cesarean delivery continues to decrease,Reference Palanisamy, Mitani and Tsen40 anesthesiologists have progressively less experience in managing the airways of pregnant women. The anesthesiologist caring for the patient undergoing fetal intervention, on the other hand, has the unique challenge of facing the obstetric airway on a relatively frequent basis. Thus, maternal-fetal anesthesiologists must be familiar with the changes in the airway during pregnancy to facilitate the often dynamic airway management required in cases ranging from sedation to planned general anesthesia. Anatomic and physiologic factors affecting the obstetric airway are listed in Table 1.2. Upper airway edema occurs in normal pregnancy as a result of capillary engorgement in the laryngeal, nasal, and oropharyngeal mucosa.Reference Leontic41 Pathologic conditions such as pre-eclampsia can exacerbate this edema.Reference Munnur and Suresh42 Changes in estrogen levels can affect nasal mucosa leading to rhinitis and epistaxis.Reference Wise, Polito and Krishnan43 The thoracic cavity also undergoes mechanical changes related to the hormone relaxin, leading to increases in the circumference of the chest wall of 5–7 cm by term.Reference Contreras, Gutiérrez and Beroíza44
| Anatomic and physiologic factors affecting the obstetric airway | |
|---|---|
| Upper airway edema | Decreased functional residual capacity |
| Breast enlargement | Increased oxygen consumption |
| Weight gain | Increased risk of aspiration |
| Cephalad displacement of diaphragm | Cricoid pressure may worsen view |
The Obstetric Anaesthetists’ Association and Difficult Airway Society published joint guidelines for the management of difficult and failed tracheal intubation for obstetrics in 2016.Reference Mushambi, Kinsella and Popat45 Although it is beyond the scope of this chapter, the algorithm is an excellent resource for the anesthesiologist caring for women undergoing fetal intervention.
Lung Mechanics
During pregnancy, diaphragmatic excursion is the primary contributor to inspiration. This results from the higher resting position combined with increased excursion distance, as well as the limitation of thoracic expansion beyond its already increased resting position.Reference Grenville-Mathers and Trenchard46
Most measures of air flow including the one-second forced expiratory volume (FEV1), forced vital capacity (FVC), and their ratio (FEV1/FVC) are largely unchanged throughout pregnancy.Reference O’Day24, Reference Russell and Chambers47 This contrasts with static lung volumes, which are altered (Table 1.3). These changes are shown in comparison with the volumes in the nonpregnant patient in Figure 1.1. The decline in functional residual capacity reaches 80% of the pre-pregnancy value at term, and is worsened in the supine position to just 70% of baseline.Reference Alaily and Carrol48, Reference Gee, Packer, Millen and Robin49 Adjusting the patient to a 30 degree head up position can increase the supine functional residual capacity (FRC) by 10%.Reference Hignett, Fernando and McGlennan50
Table 1.3 Lung and chest wall mechanics in pregnancy
| Parameter | Change |
|---|---|
| Diaphragm excursion | ↑ |
| Chest wall excursion | ↓ |
| Tidal volume | ↑ |
| Minute ventilation | ↑ |
| VC | ↔ |
| FEV1 | ↔ |
| FEV1/FVC | ↔ |
| Closing capacity | ↔ |
VC, vital capacity; FEV1, forced expiratory volume in one second; FVC, forced vital capacity
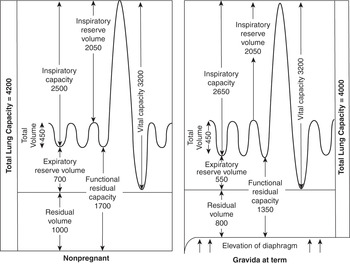
Figure 1.1 Lung volume changes during pregnancy.
Gas Exchange and Arterial Blood Gases
During pregnancy, minute ventilation increases as a result of a 33% increase in tidal volume and an increase in respiratory rate of one to two breaths per minute.Reference Vered, Mark Poler, Gibson, Wlody and Pérez5Reference Robson, Dunlop, Moore and Hunter1 These changes, which are related to the direct respiratory stimulant properties of progesterone,Reference Zwillich, Natalino, Sutton and Weil52 occur during the first trimester and do not change significantly throughout the remainder of pregnancy. Up to 75% of women experience symptoms of dyspnea related to awareness of this increased ventilation.Reference Vered, Mark Poler, Gibson, Wlody and Pérez5Reference Simmons, Gillin and Jeremy3
The increase in minute ventilation leads to a slight respiratory alkalosis despite a 30% increase in carbon dioxide production during pregnancy, primarily related to the increased metabolic rate of the fetus. Compared to nonpregnant adults, the PaCO2 decreases, leading to pH increases.Reference Abbassi-Ghanavati, Greer and Cunningham54 The gradient between PaCO2 and end tidal CO2 that exists in most patients is absent or reversed in many pregnant women, likely because of the increased cardiac output and decreased alveolar dead space that occur during pregnancy.Reference Shankar, Moseley, Vemula, Ramasamy and Kumar55 The decline in PaCO2 also leads to a slight increase in PaO2 initially. As the pregnancy progresses, FRC may be below closing capacity and the PaO2 can drop below 100 mmHg. Normal blood gas values by trimester are shown in Table 1.4. The anesthesiologist caring for a pregnant patient can use position adjustments such as lateral decubitus to reduce shunting that occurs in the supine position as a result of increased abdominal pressure elevating the diaphragm. This decrease in shunt reduces the alveolar to arterial oxygen gradient, improving oxygen transfer to the fetus.
Table 1.4 Blood gas values during pregnancy
| Nonpregnant adult | First trimester | Second trimester | Third trimester | |
|---|---|---|---|---|
| pH | 7.4 | 7.44 | 7.44 | 7.44 |
| pO2 (mmHg) | 90–100 | 93–107 | 90–105 | 92–107 |
| pCO2 (mmHg) | 38–42 | 30 | 30 | 25–33 |
| Bicarbonate (mEq/L) | 22–26 | 21 | 20 | 16–22 |
Neurologic System
Anatomy
Anatomic changes affecting the spine during pregnancy are an important consideration for anesthesiologists caring for patients undergoing fetal intervention. The epidural space, containing both epidural fat and veins, enlarges during pregnancy. Cerebrospinal fluid volume decreases.Reference Hirabayashi, Shimizu, Fukuda, Saitoh and Igarashi56
Over 50% of pregnant women complain of low back pain, with onset most commonly in the third trimester.Reference Ansari, Hasson, Naghdi, Keyhani and Jalaie57 In a study of women of childbearing age, MRI revealed a disc bulge or herniation in 53% of pregnant and 54% of nonpregnant women.Reference Weinreb, Wolbarsht, Cohen, Brown and Maravilla58 Lumbar disc bulge or herniation is not a contraindication to neuraxial analgesia or anesthesia.
Central Nervous System
During pregnancy, cerebrovascular resistance decreases, causing an increase in cerebral blood flow from 44 mL/min/100 g in the first trimester to 52 mL/min/100 g in the third trimester.Reference Nevo, Soustiel and Thaler59 The decreased cerebrovascular resistance, along with increased hydrostatic pressure, also leads to increased permeability of the blood-brain barrier.Reference Johnson and Cipolla60 In normal pregnancies autoregulation is preserved or slightly improved, but in pathophysiologic states such as preeclampsia, autoregulation is abnormal and does not necessarily correlate with blood pressure abnormalities.Reference van Veen, Panerai, Haeri, Griffioen, Zeeman and Belfort61
As women approach term, elevated levels of endorphins and enkephalins are found in the plasma and CSF. Although a causation mechanism is unclear, the pain threshold increases concurrently with these changes.Reference Cogan and Spinnato62, Reference Abboud, Sarkis and Hung63 Pregnant women experience more sleep disturbances including insomnia, daytime sleepiness, snoring, and transient restless leg syndrome.Reference Manconi, Govoni and De Vito64 These symptoms are caused by mechanical as well as hormonal factors, particularly related to progesterone.Reference Pien and Schwab65
Hematologic System
The anesthesiologist caring for the obstetric patient must be familiar with the myriad of changes in the hematologic system during pregnancy. The increased blood flow to the uterus puts the patient at increased risk of hemorrhage during fetal intervention, and alterations in baseline blood components and coagulation factors in pregnant women have important implications for management should hemorrhage occur.
Blood Volume
Total plasma volume increases throughout pregnancy, starting in the first trimester with a 10–15% increase.Reference Bernstein, Ziegler and Badger66 By term, pregnant women have a plasma volume of 30–50% above nonpregnant levels.66–Reference Pritchard68 Based on plasma renin and atrial natriuretic peptide levels, this increase in plasma volume seems to be in response to systemic vasodilation and increased vascular capacitance rather than a primary blood volume expansion.Reference Schrier and Fassett69,Reference Nadel, Ballermann, Anderson and Brenner70
Red Blood Cells
Red blood cell mass increases in pregnancy, reaching 20–30% above baseline levels at term. The hormonal regulation of this increase is complex: erythropoietin increases from baseline, human placental lactogen augments the action of erythropoietin, estrogen inhibits erythropoietin, and progesterone negates the activity of estrogen on erythropoietin.Reference Peck and Arias71 Despite the increase in red blood cell mass, “dilutional anemia,” or physiologic anemia of pregnancy, results from the greater relative increase in plasma volume. Even healthy pregnant women should receive iron supplementation to support this increased erythrocyte production. The nadir of this physiologic anemia occurs between 28 and 36 weeks’ gestation.Reference Whittaker, Macphail and Lind72 Despite the difficulty of determining when anemia in pregnancy becomes pathologic, the Centers for Disease Control and Prevention has defined anemia as a hemoglobin less than 11 g/dL in the first and third trimesters and less than 10.5 g/dL in the second trimester.73 The Institute of Medicine has recommended decreasing these thresholds by 0.8 g/dL for African-American adults.Reference Earl and Woteki74 Women with hemoglobin levels below these cutoffs should undergo evaluation.
White Blood Cells
An increased level of neutrophils leads to leukocytosis in pregnancy. The rise in white blood cell count begins in the first trimester and plateaus in the second or third trimester between 9,000 and 15,000 cells/μL.Reference Kuvin and Brecher75 During labor, leukocytosis can become more marked, increasing to as high as 29,000 cells/μL.Reference Molberg, Johnson and Brown76,Reference Acker, Johnson, Sachs and Friedman77
Platelets
Thrombocytopenia in pregnancy is of special concern to the anesthesiologist, especially when considering neuraxial anesthesia or analgesia. Although the exact mechanisms are not completely understood, some etiologies include pregnancy-related pathology such as hypertensive disorders of pregnancy, idiopathic hematologic disorders such as idiopathic thrombocytopenic purpura, or gestational thrombocytopenia. In the setting of thrombocytopenia, both the absolute platelet level and the trend over time contribute to management decisions. Many obstetric anesthesiologists have a “cutoff” for consideration of neuraxial placement at around 70,000 platelets/μL, but this arbitrary cutoff level would be impacted by the risk-benefit ratio for a neuraxial analgesic or anesthetic technique, as well as the trend in the platelet count and overall clotting function demonstrated by thromboelastography.Reference Camann78
Coagulation
Circulating levels of multiple coagulation factors change during pregnancy, leading to an overall hypercoagulable state. As a result of hypercoagulability, pregnant women are at increased risk for venous thromboembolism (VTE); VTE has been implicated in 13–15% of maternal deaths in developed countries.Reference Khan, Wojdyla, Say, Gulmezoglu and Van Look79, Reference Say, Chou and Gemmill80 For this reason, increased emphasis is being placed on decreasing maternal morbidity and mortality related to embolic disease.Reference D’Alton, Friedman and Smiley81 As guidelines change, it is likely that increasing numbers of pregnant women will receive anticoagulants as prophylaxis against VTE. Although multiple major societies have published guidelines for VTE prophylaxis, recommendations differ, and a task force has formed to define a consensus bundle.Reference Palmerola, D’Alton, Brock and Friedman82, Reference D’Alton, Friedman and Smiley83 Given the often unplanned nature of fetal intervention, patients who are receiving ongoing VTE prophylaxis are likely to present for surgery. The implications of anticoagulation on obstetric anesthesia management are significant; therefore, anesthesiologists must carefully consider these medications when electing a neuraxial technique for analgesia or anesthesia.
Table 1.5 shows expected laboratory values in pregnancy.Reference Abbassi-Ghanavati, Greer and Cunningham54 Thromboelastography demonstrates changes associated with hypercoagulability in pregnancy (Figure 1.2). These alterations have implications for management of obstetric hemorrhage. While details on the management of hemorrhage are beyond the scope of this chapter, the anesthesiologist caring for a woman undergoing fetal intervention should be familiar with the differences in the coagulation system, particularly with relation to fibrinogen stores and antifibrinolysis,Reference Shakur, Roberts and Fawole84 during pregnancy to facilitate appropriate care in the event of large volume blood loss.
Table 1.5 Hematologic laboratory values during pregnancy
| Nonpregnant adult | First trimester | Second trimester | Third trimester | |
|---|---|---|---|---|
| Hemoglobin (g/dL) | 12–15.8 | 11.6–13.9 | 9.7–14.8 | 9.5–15 |
| Hematocrit (%) | 35.4–44.4 | 31–41 | 30–39 | 28–40 |
| White blood cells (×103/mm3) | 3.5–9.1 | 5.7–13.6 | 5.6–14.8 | 5.9–16.9 |
| Platelets (×109/L) | 165–415 | 174–391 | 155–409 | 146–429 |
| Fibrinogen (mg/dL) | 233–496 | 244–510 | 291–538 | 373–619 |
| Partial thromboplastin time, activated (sec) | 26.3–39.4 | 24.3–38.9 | 24.2–38.1 | 24.7–35 |
| Prothrombin time (sec) | 12.7–15.4 | 9.7–13.5 | 9.5–13.4 | 9.6–12.9 |
| INR | 0.9–1.04 | 0.89–1.05 | 0.85–0.97 | 0.8–0.94 |

Figure 1.2 Comparative thromboelastographs in nonpregnant (Group I), nonlaboring term pregnant (Group II), and laboring (Group III) women.
Gastrointestinal System
Gastrointestinal Changes
As the uterus transitions from a pelvic to an abdominal organ, the stomach is displaced upward and leftward, rotating about 45 degrees to the right relative to its normal vertical position.Reference Gaiser and Chestnut27 This change in position displaces the esophagus upward, with the uppermost intra-abdominal portion moving into the thorax. The tone of the lower esophageal high-pressure zone decreases, leading to higher prevalence of gastric reflux.Reference Van Thiel, Gavaler and Stremple85 Between one-third and one-half of pregnant women complain of gastroesophageal reflux disease, with increasing prevalence as the pregnancy progresses.Reference Richter86
Studies evaluating gastric acid secretion and gastric pH in pregnancy have conflicting results. However, studies using various methodologies have consistently shown that pregnancy does not alter gastric emptying;Reference Gaiser and Chestnut27 and in addition, gastric emptying does not differ between obese and lean patients.Reference Wong, McCarthy, Fitzgerald, Raikoff and Avram87 On the other hand, esophageal and intestinal transit times are both slowed during pregnancy.Reference Chiloiro, Darconza, Piccioli, De Carne, Clemente and Riezzo88, Reference Derbyshire, Davies and Detmar89 About 80% of women experience nausea and vomiting in pregnancy, usually starting early in the first trimester and occasionally lasting until 12–16 weeks’ gestation.Reference Gill, Maltepe and Koren90 Constipation is also a common complaint of pregnant women.Reference Costantine91
Liver and Gallbladder Changes
The liver’s position in the abdomen changes to be more superior, posterior, and rightward during pregnancy. Liver function tests, including bilirubin, transaminases, and lactate dehydrogenase, remain within the normal nonpregnant limits during pregnancy. Alkaline phosphatase, which is also produced by the placenta, increases twofold.Reference Abbassi-Ghanavati, Greer and Cunningham54
The risk of gallbladder dysfunction increases during pregnancy, with a 5–12% incidence of gallstones.Reference Mendez-Sanchez, Chavez-Tapia and Uribe92 Cholecystectomy is one of the most common non-obstetric surgeries indicated during pregnancy.
Renal System
Renal blood flow and glomerular filtration rate increase by 50% by the beginning of the second trimester, likely because of vasodilation of both afferent and efferent arterioles.Reference Davison and Dunlop93 Serum creatinine is thus decreased, with levels greater than 0.8 mg/dL indicating possible renal dysfunction.Reference Mattison94 Hormonal changes also lead to sodium retention in pregnancy, with a consequent increase in total body water by up to 8 L, including that distributed as 1.5 L in plasma volume and 3.5 L in the fetus, placenta, and amniotic fluid.Reference Costantine91 Anesthesiologists caring for pregnant women must consider alterations in renal function and increased volume of distribution when administering medications that are cleared in the urine or that are hydrophilic.
The kidney compensates to maintain the acid–base status during pregnancy. The chronic respiratory alkalosis of pregnancy leads to a compensatory increase in the renal excretion of bicarbonate.Reference O’Day24 This decrease in serum bicarbonate impacts the pregnant patient’s buffering capability when faced with an acid load.Reference Gaiser and Chestnut27
Both progesterone and relaxin affect smooth musculature in the urinary system, causing dilation of the collecting system and urinary stasis.Reference Rasmussen and Nielsen95 The hydronephrosis-related urinary stasis as well as ureterovesical reflux related to decreased ureteral tone and increased rates of glycosuria which encourage bacterial growth, result in pregnant women having a higher likelihood of urinary tract infections.Reference Rasmussen and Nielsen95,Reference Delzell and Lefevre96
Endocrine System
Many women of childbearing age either have or are at risk of hypothyroidism during pregnancy.Reference Dichtel and Alexander97 One study showed a 15% rate of gestational hypothyroidism in pregnant women, with one-third of these women being symptomatic.Reference Blatt, Nakamoto and Kaufman98 Clinical diagnosis is difficult, as many symptoms of hypothyroidism mimic common symptoms of pregnancy, so evaluation of hormone levels is essential for diagnosis during pregnancy.
The changes in estrogen levels during pregnancy cause thyroid-binding globulin to increase, with resultant increases in total triidodothyronine (T3) and thyroxine (T4). Normally, the concentrations of free T3 and T4 remain unchanged, but the measurement of these levels can be unreliable in pregnancy.Reference Harada, Hershman and Reed99, Reference Dichtel and Alexander97 For this reason, thyroid stimulating hormone (TSH) is the gold standard of thyroid function evaluation during pregnancy. Of note, placental human chorionic gonadotropin (hCG) lowers TSH levels, necessitating pregnancy-specific ranges for evaluation.Reference Dichtel and Alexander97
Hormonal alterations in pregnancy also result in insulin resistance. Despite a more robust insulin response, blood glucose levels are higher after a carbohydrate load during pregnancy than baseline. Pregnant women are also more prone to ketosis in response to periods of fasting.Reference Fisher, Sutherland and Bewsher100
Musculoskeletal System
Relaxin, a hormone produced by both the corpus luteum and the placenta, affects collagen remodeling in the pregnant patient.Reference Kristiansson, Nilsson-Wikmar, Von Schoultz, Svardsudd and Wramsby101 Joint mobility increases via relaxin as well as biomechanical strain from the pregnancy itself.Reference Berg, Hammar, Möller-Nielsen, Lindén and Thorblad102 Changes in posture, such as exaggeration of lumbar lordosis, can also lead to nerve injury. Lateral femoral cutaneous nerve stretching can result in meralgia paresthetica; brachial plexus neuropathies can occur from postural alterations as well.Reference Gaiser and Chestnut27
About half of women have back pain during pregnancy, with rates increasing as they approach full term.Reference Ansari, Hasson, Naghdi, Keyhani and Jalaie57 Anesthesiologists are often asked about back pain in relation to neuraxial anesthesia/analgesia. Patients should be reassured that neuraxial interventions do not increase the likelihood of chronic back pain; multiple prospective reports and randomized controlled trials have failed to show a link between epidural use and long-term back pain.Reference Loughnan, Carli, Romney, Doré and Gordon103–Reference Breen, Ransil, Groves and Oriol105 Furthermore, while patients with baseline back pain are more likely to have continued or progressive back pain related to pregnancy, surgery, and delivery with the associated limitations on mobility, preexisting low back pain is not a contraindication to neuraxial analgesia or anesthesia.Reference Howell, Kidd and Roberts106,Reference Russell, Dundas and Reynolds104,Reference Loughnan, Carli, Romney, Doré and Gordon103
Conclusion
As fetal intervention becomes more widely practiced, the field of maternal-fetal anesthesia is rapidly expanding. The maternal-fetal anesthesia specialist has the unique task of caring for two patients, both of whom are undergoing rapid and significant anatomic and physiologic changes. Optimal anesthetic care during a fetal intervention and beyond necessitates a clear understanding of all aspects of the highly dynamic physiology of pregnant women.