Introduction
According to Joint United Nations Program on HIV/AIDS (UNAIDS) there were 190 000 children (170 000–220 000) 0–14 years of age living with HIV in Uganda (Joint United Nations Program on HIV/AIDS, 2015). As a result of the national scale-up of antiretroviral therapy (ART), the number of children living with HIV is increasing (UNAIDS, 2013), which highlights the importance of being able to identify those at higher risk of developing complications associated with HIV infection, such as neurobehavioral deficits (Laughton et al. Reference Laughton, Cornell, Boivin and Van Rie2013).
HIV is a neurotropic virus that invades the central nervous system (CNS) early after infection, primarily via infected monocytes/macrophages and CD4+ lymphocytes (Kovacs Reference Kovacs2009). The psychological effects of HIV on children are frequent, ranging from mild to severe (Mellins et al. Reference Mellins, Kang, Leu, Havens and Chesney2003; Wolters & Brouwers, Reference Wolters and Brouwers2005; Nachman et al. Reference Nachman, Chernoff, Williams, Hodge, Heston and Gadow2012). Developmental deficits among HIV-1 infected children include: language and motor skills (Ultmann et al. Reference Ultmann, Diamond, Ruff, Belman, Novick, Rubinstein and Cohen1987; Drotar et al. Reference Drotar, Olness, Wiznitzer, Guay, Marum, Svilar and Kiziri-Mayengo1997), verbal and memory deficits (Levenson et al. Reference Levenson, Mellins, Zawadzki, Kairam and Stein1992), visual spatial integrative ability (Boivin et al. Reference Boivin, Green, Davies, Giordani, Mokili and Cutting1995; Laughton et al. Reference Laughton, Cornell, Boivin and Van Rie2013) and executive functions (Bisiacchi et al. Reference Bisiacchi, Suppiej and Laverda2000). In addition, they are at higher risk of psychiatric and mental health problems (Laughton et al. Reference Laughton, Cornell, Boivin and Van Rie2013), delinquent behavior and poor social competence (Bomba et al. Reference Bomba, Nacinovich, Oggiano, Cassani, Baushi, Bertulli and Badolato2010). Although dementia and encephalopathy are now rare in developing countries with the introduction of antiretrovirals (ART) (Ghafouri et al. Reference Ghafouri, Amini, Khalili and Sawaya2006), cognitive problems, developmental delays, behavioral, psychiatric and motor problems are still common among children living with HIV who have been infected perinatally. Research suggests that this might be related to host-related factors, including T-cell activation (Jennings et al. Reference Jennings, Rich, Siegel and Landay1994). However, other biological and environmental causes should also be considered as potential risk factors for poor behavioral outcomes (Mellins & Malee, Reference Mellins and Malee2013; Llorente et al. Reference Llorente, Brouwers, Leighty, Malee, Smith, Harris and Chase2014).
HIV biomarkers (e.g. viral load and HIV-1 subtype) and immunological parameters (e.g. CD4+ and CD8+ T-cell count) have been identified as independent markers of disease progression (Sanchez-Ramon et al. Reference Sanchez-Ramon, Bellon, Resino, Canto-Nogues, Gurbindo, Ramos and Munoz-Fernandez2003; Sacktor et al. Reference Sacktor, Nakasujja, Skolasky, Rezapour, Robertson, Musisi and Quinn2009; Boivin et al. Reference Boivin, Ruel, Boal, Bangirana, Cao, Eller and Wong2010; Tobin & Aldrovandi, Reference Tobin and Aldrovandi2013). Higher HIV viral loads have been associated to poor neuropsychological function among children and adolescents (Jeremy et al. Reference Jeremy, Kim, Nozyce, Nachman, McIntosh, Pelton and Stanley2005), while CD4+ and CD8+ T-cells are fundamental for HIV infection control, with their efficiency fluctuating from time of infection and specific type of surface receptors (Appay & Sauce, Reference Appay and Sauce2008). Higher CD4+ and CD8+ T- cell counts have been associated with better neurobehavioral functioning (Marcotte et al. Reference Marcotte, Deutsch, McCutchan, Moore, Letendre, Ellis and Grant2003; Sanchez-Ramon et al. Reference Sanchez-Ramon, Bellon, Resino, Canto-Nogues, Gurbindo, Ramos and Munoz-Fernandez2003). In general, CD8+ CD38+ HLADR+ subsets, which are markers of activated CD8+ T-cells, are observed in more advanced disease stages (McCloskey et al. Reference McCloskey, Kohn, Lesser, Bakshi and Pahwa2001). Kapetanovic et al. reported in 2012 that higher CD4+ CD38+ HLADR+ T-cells had a neuroprotective effect in HIV infected children younger than 1 year of age (Kapetanovic et al. Reference Kapetanovic, Aaron, Montepiedra, Burchett and Kovacs2012). Mekmullica et al. reported a favorable association between low immune activation and the psychomotor developmental index of the Bayley scales of infant development through the third year of life, as measured by ≤5% CD8+HLA-DR+ among HIV infected children <2 months (Mekmullica et al. Reference Mekmullica, Brouwers, Charurat, Paul, Shearer, Mendez and Smith2009).
Recent research provides evidence of behavioral problems associated with attention among HIV infected children (Mellins et al. Reference Mellins, Elkington, Leu, Santamaria, Dolezal, Wiznia and Abrams2012). Additionally, compromised executive function and slowed information processing have also been reported (Wachsler-Felder & Golden, Reference Wachsler-Felder and Golden2002). Such findings underscore the importance of examining behavior, in addition to cognition in children living with HIV. However, it is important to highlight that research aimed at identifying risk factors for behavioral problems among this population has shown mixed results and are limited in their comparability (Mellins & Malee, Reference Mellins and Malee2013).
Several tools have been used to measure behavioral problems in children living with HIV (Black et al. Reference Black, Baqui, Zaman, McNary, Le, Arifeen and Black2007) some of them tested and validated for their use in sub-Saharan Africa (Bomba et al. Reference Bomba, Nacinovich, Oggiano, Cassani, Baushi, Bertulli and Badolato2010; Tadesse et al. Reference Tadesse, Berhane Tsehay, Girma Belaineh and Alemu2012; Boivin et al. Reference Boivin, Bangirana, Nakasujja, Page, Shohet, Givon and Klein2013). The Behavior Rating Inventory of Executive Function (BRIEF) (Gioia et al. Reference Gioia, Isquith, Guy and Kenworthy2000) and the Achenbach Child Behavior Checklist (CBCL) (Achenbach, Reference Achenbach1991) are among the most widely used measures to assess behavioral, emotional, social and functional problems in school aged children. The BRIEF was designed to screen for emotional and behavioral aspects of a child's executive functioning as assessed from the perspective of the principal caregiver or parent. In contrast, the CBCL was designed to more broadly screen for emotional and behavioral psychiatric symptoms in children.
Given the important behavioral problems identified among HIV infected children and the clinical relevance associated to CNS involvement, our objective was to identify viral and immunological biomarkers of HIV disease associated with poor neurodevelopmental outcomes among school-aged children living in Kayunga, Uganda, as measured by the BRIEF score and the CBCL.
Methods
Study design
This is a secondary data analysis of baseline data collected from children aged 5–12 years participating in a randomized-controlled trial exploring the effects of a Computerized Cognitive Rehabilitation Training (CCRT) program for HIV infected children aiming to evaluate the effectiveness of CCRT in improving the cognitive performance outcomes and reducing the psychiatric symptoms among Ugandan children living with HIV. Participants were screened and recruited in Kayunga district (80 km northeast of Kampala) between 2010 and 2013. Children with a medical history of serious birth complications, severe malnutrition, bacterial meningitis, encephalitis, cerebral malaria, or other known brain injuries or disorders requiring hospitalization were excluded. A total of 144 HIV infected children were enrolled at baseline. All children were perinatally-infected and confirmed as HIV-positive with a Western Blot or ELISA test.
Our aim was to identify the viral and immunological biomarkers of HIV disease associated with poor neurodevelopmental outcomes among school-aged children living in Kayunga, Uganda as measured by the BRIEF score and the CBCL.
Study procedures
Written consent was obtained from the parent/guardian and assent from children 7 years and older. After administering the informed consent, child testing and caregiver questionnaires were done in Luganda, the local language spoken in Kayunga district, in a private and quiet setting inside the project's office. Peripheral blood draws were performed in all children to determine complete blood cell count and immunologic biomarkers, including CD4+ and CD8+ T- cell counts and viral load. Blood specimens taken at Kayunga District Hospital were transported to the Makerere University-Walter Reed Project (MU-WRP).
The Institutional Review Boards of Michigan State University, University of Michigan, the School of Medicine Research Ethics Committee at Makerere University, and the Ugandan National Council for Science and Technology approved the study.
Viral load assessment
Viral load was performed using the Amplicor HIV-1 Monitor test, version 1.5 (Roche Diagnostics, Indianapolis, IN) in the standard mode.
Assessment of CD4+ and CD8+ T-cell activation
Imunophenotyping of CD4+ and CD8+ T-cells was performed. Samples were processed at MU-WRP. The current analysis focuses on two phenotypes: CD4+ CD38+ HLADR+ and CD8+ CD38+ HLADR+.
To measure immune activation, peripheral blood mononuclear cells (PBMCs) were isolated by ficoll-hypaque gradient centrifugation. Freshly isolated PBMCs were analyzed for CD4+ and CD8+ T cell activation using the Beckton-Dickinson FACScalibur (BD, San Carlos, California). Cells were stained with fluorochrome monoclonal antibodies: CD4+ or CD8+ PerCP-cy5.5, HLA-DR PE and CD38 APC. Preset gating was applied to all samples based on a naturally occurring break in the expression pattern of the activation markers documented in healthy Ugandan children.
Behavioral assessments
The CBCL for school-age children (6–18 years) was administered to the principal caregiver. It is a paper-pencil parent/caregiver report on child behavior consisting of 120 items scores on a three-point Likert scale (0 = absent, 1 = occurs sometimes, 2 = occurs often). The time frame for the item responses is the past 6 months. The instrument is organized in 8 syndrome scales (Anxious/Depressed, Depressed, Somatic Complaints, Social Problems, Attention Problems, Thought Problems, Rule-Breaking Behavior, Aggressive Behavior) that group into higher order factors-internalizing (emotional problems) and Externalizing Problems [EP (behavioral problems)], respectively, and into a summary score; Total Problems [TP (internalizing plus externalizing, plus sleep and other Problems)]. It has been widely used as rating scale in different context including Ugandan children, where test-retest and internal reliability were 0.64 and 0.83, respectively (Bangirana et al. Reference Bangirana, Nakasujja, Giordani, Opoka, John and Boivin2009a , Reference Bangirana, Nakasujja, Giordani, Opoka, John and Boivin b ). The CBCL was translated into Luganda by a research assistant and then back-translated to English by a different research assistant, both fluent in Luganda and English. A psychiatrist fluent in both Luganda and English compared the two English versions and resolved any discrepancies by editing the translated version to match the original English version. However, the translation was not checked nor authorized by the authors of the CBCL. Cross-cultural norms are available for the CBCL, which were applied to obtain the T-scores reported in our study. Additionally, the CBCL has proven sensitive to exposure and intervention effects of pediatric HIV in out other published work (Bangirana et al. Reference Bangirana, Nakasujja, Giordani, Opoka, John and Boivin2009a , Reference Bangirana, Nakasujja, Giordani, Opoka, John and Boivin b ; Boivin et al. Reference Boivin, Bangirana, Nakasujja, Page, Shohet, Givon and Klein2013).
Caregivers were also interviewed with the BRIEF for school-aged children. This instrument is specifically designed to measure the range of executive function behaviors in children with 86-items in 8 non-overlapping clinical scales (Inhibit, Shift, Emotional Control, Initiate, Working Memory, Plan/Organize, Organization of Materials and Monitor). The BRIEF is divided into two broad indexes, each of which is derived from specific clinical scales; Metacognition Index (Monitor, Organization of Materials, Plan/Organize, Working Memory, Initiate) and Behavioral Regulation (Emotional Control, Shift, Inhibit). A Global Executive Composite score takes into account all clinical scales and represents the child's overall executive function. Publisher copyright permission was obtained for the BRIEF and was translated, as specified by the publisher (PAR, Inc.). The final version was approved by one of the test authors (Peter Isquith) after several iterations.
Statistical analyses
Demographic characteristics and symptom endorsement frequencies were calculated. Internal consistency of the BRIEF and CBCL scales were evaluated using Cronbach’s α. Simple- (SLR) and multiple-linear regression (MLR) modeling was used to relate BRIEF and CBCL scores to demographic characteristics (sex and age), ART status, viral load and immunologic biomarkers (CD4+ cell count, CD4+ CD38+ HLADR+ and CD8+ CD38+ HLADR+). All statistics were two-sided. Models for MLR were constructed on the basis of statistical significance (p < 0.05) of the association between outcome and variable using SLR, and were added to the final model using the forward stepwise selection method. All analyses were performed in STATA version 12 [Stata (computer program). Version 12. College Station, TX 2012].
Results
A total of 144 HIV infected children underwent neuropsychological assessment as part of the parent study. Table 1 presents the demographic characteristics of the study sample and summarizes their immunological parameters and neuropsychological test-scores. The mean age of the study sample was 8.9 years (s.d. = 1.9 years) and gender distribution was equal (50% male). Participants’ mean CD4+ CD38+ HLADR+ T-cell activation was 5.7% (s.d. = 5.1%), CD 8+ CD38+ HLADR+ T-cell activation was 17.5% (s.d. = 11.2%). CD4 cell % mean was 35.1% (s.d. = 15.0%), one child had a viral load of <50 copies/ml and 38 participants (26.4%) had plasma HIV RNA of >100 000 copies/ml (see Table 1).
Table 1. Demographic characteristics, immunological parameters and CBCL and BRIEF scores of school-age children living with HIV in Kayunga, Uganda
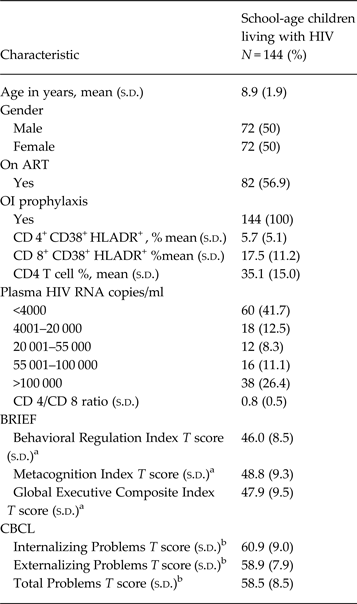
OI, opportunistic infections; ART, antiretroviral therapy.
a Behavior Rating Inventory of Executive Function (BRIEF) parent form for children 5–18 years. T scores (adjusted for age and gender using American norms).
b Achenbach Child Behavior Checklist (CBCL) parent form for children 6–18 years T scores (adjusted for age and gender using cross-cultural norms).
Table 1 also shows the BRIEF and CBCL scores. Mean Global Composite Index of the BRIEF was 46 (s.d. = 9.5), while the mean TP Scale score of the CBCL was 58.5 (s.d. = 8.5). Out of 144 participants, 7 (4.9%) in the Global Executive Composite Index and 36 (25%) in the TP Scale had a score ≥65, score that is associated with higher likelihood of executive dysfunction.
Internal reliability (Cronbach's α) was 0.88 for the BRIEF and 0.76 for the CBCL.
Results from linear regression analyses are presented in Tables 2 and 3. Regression models for BRIEF were adjusted for gender, age, ART status, CD4 T-cell activation and viral load. Higher behavioral regulation index scores on the BRIEF were associated with higher viral loads (aβ = 16.7 × 10−6, 95% CI −5.00 × 10−6 to 28.4 × 10−6) (Table 2). Children who were older had lower Metacognition Index (MI) scores (e.g. less capacity to plan, working memory and organize) when compared with younger ones (aβ = −1.06, 95%CI −2.02 to −0.09) (Table 2).
Table 2. Unadjusted and adjusted linear regression models for BRIEF among 144 school-age children living with HIV in Kayunga, Uganda
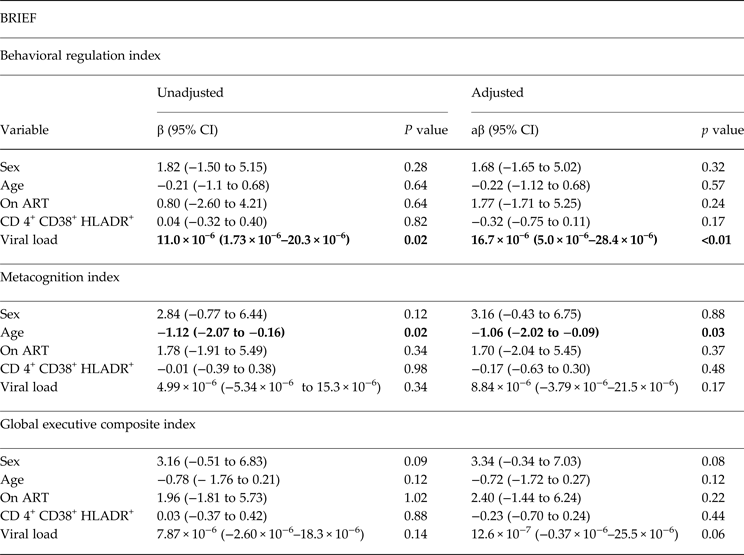
ART, antiretroviral therapy
a T scores were used for simple and multiple linear regression models.
Statistically significant values appear in bold
Table 3. Unadjusted and adjusted linear regression models for CBCL among 144 school-age children living with HIV in Kayunga, Uganda
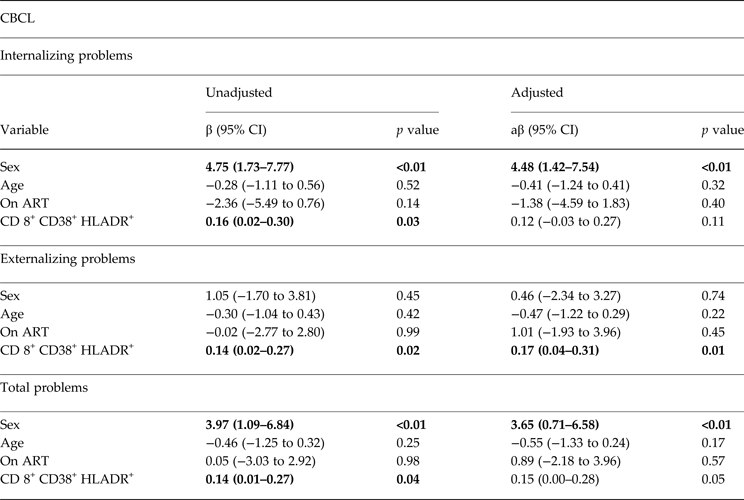
ART, antiretroviral therapy
a T scores were used for simple and multiple linear regression models.
Statistically significant values appear in bold
Regression models for CBCL were adjusted for gender, age, ART status and CD8T-cell activation. Gender was associated with higher Internalizing and TPs scores (aβ = −4.48, 95%CI −1.42 to 7.54). Girls scored on average 4.48 points higher in the Internalizing scale and 3.65 points in the TPs scale (i.e. more behavior problems) (Table 3). Higher percentage of CD8+ CD38+ HLADR+ were associated with higher scores in the EPs Scale (aβ = −0.17, 95% CI −0.04 to 0.31) and the TPs Scale (aβ = −0.15, 95% CI −0.00 to 0.28) of the CBCL (Table 3).
Discussion
This study showed that clinically stable children living with HIV in Uganda have more behavioral and emotional problems as reported by their parents in the BRIEF (executive function) and CBCL (psychiatric symptoms) questionnaires with increasing viral loads and higher percentages of CD8+ T-cells expressing CD38+ and HLADR+. To the best of our knowledge, this study represents one of the first to evaluate the extent to which the immunological features of HIV disease are predictive of behavioral and psychiatric symptoms in rural school-age children in a sub-Saharan African setting. These findings are comparable with studies in the USA and elsewhere demonstrating observable behavioral sequelae of HIV disease in young children (Mellins et al. Reference Mellins, Kang, Leu, Havens and Chesney2003; Wolters & Brouwers, Reference Wolters and Brouwers2005).
The strongest correlates of increased behavioral symptoms in this study were CD8+ T-cells expressing CD38+ and HLADR+ and viral load, other factors were consistent with previous studies that have reported demographic characteristics, such as gender, to be strongly correlated with behavioral problems (Mellins et al. Reference Mellins, Smith, O'Driscoll, Magder, Brouwers, Chase and Matzen2003).
We found that parents are more likely to report Internalizing Problems for girls than boys for the CBCL, which is consistent with what has been reported in most countries (Rescorla et al. Reference Rescorla, Achenbach, Ginzburg, Ivanova, Dumenci, Almqvist and Domuta2007), but in disagreement with other studies reporting no gender differences in pre-school children (Keiley et al. Reference Keiley, Bates, Dodge and Pettit2000; Shala & Dhamo, Reference Shala and Dhamo2013). Regarding age, we observed lower values with increasing age on the MI of the BRIEF values (i.e. lower levels of executive dysfunction), corresponding with the development of cognitive abilities such as memory, comprehension and learning processes taking place from pre-school to adolescence (Händel et al. Reference Händel, Artelt and Weinert2013).
Although the pathogenic mechanism of CNS disease is not fully understood, immune activation seems to play a fundamental role, with CD8+ T-cells continually crossing the blood-brain barrier and promoting activation of infected cells, which in turn lead to a pro-inflammatory cascade, resulting in further damage (Kovacs, Reference Kovacs2009). Research suggests that having higher CD8+ CD38+ HLADR+ cells is associated with having poorer neurobehavioral outcomes (McCloskey et al. Reference McCloskey, Kohn, Lesser, Bakshi and Pahwa2001). Children with higher percentages of CD8+ T-cells expressing CD38+ and HLADR+ had more psychiatric symptoms (higher scores) in the EPs and in the TPs scale of the CBCL, suggesting poorer emotional and behavioral outcomes. We found no association between CD4+ CD38+ HLADR+ and the BRIEF or the CBCL.
More psychiatric symptoms in the EPs and TPs scales of the CBCL were detected in children who had a higher percentage of CD8+ CD38+ HLADR+. These findings are consistent with previous research reported by Mekmullica et.al. who found that the absence of immune activation as measured by having ≤5% CD8+ HLADR+ cells was associated with better performance on the Psychomotor Developmental Index of the Bayley scale through the third year of life (Mekmullica et al. Reference Mekmullica, Brouwers, Charurat, Paul, Shearer, Mendez and Smith2009). However, this is inconsistent with research by Kapetanovic et.al. who suggested that CD8+ CD38+ HLADR+ cells had a neuroprotective effect among children <1 year of age (Kapetanovic et al. Reference Kapetanovic, Aaron, Montepiedra, Burchett and Kovacs2012). Our findings suggest that school aged children with higher percentage of CD8+ CD38+ HLADR+ cell activation are more likely to have a negative behavioral outcomes as measured by the CBCL which is consistent with the concept that CD38+ and HLADR+ are observed in advanced disease stages (McCloskey et al. Reference McCloskey, Kohn, Lesser, Bakshi and Pahwa2001).
Viral load has been previously described as a factor that impacts a child's behavior. Jeremy et.al. reported poor neuropsychological functioning in HIV infected children with high viral loads (>50 000 copies/ml), specifically, having a high viral load was associated with difficulties on children's emotional control, task shifting and inhibition (Jeremy et al. Reference Jeremy, Kim, Nozyce, Nachman, McIntosh, Pelton and Stanley2005). Similarly, in our study, higher viral loads were associated with higher scores on the Behavior Regulation Scale of the BRIEF, encompassing difficulties with emotional control, task shifting and inhibition. Although the magnitude of this increment on the Behavior Regulation Scale was small, we think that this could have clinical significance that could guide preventive interventions and treatment services.
Further research is needed to elucidate the immunopathological pathways through which executive function, as collected on the BRIEF, can be affected by HIV, which could specifically be associated with HIV cognitive impairment. Longitudinal studies could contribute to our understanding on how ART and viral load fluctuations across time can affect behavior in children.
In contrast to previous findings, we did not find an association between CD4+ CD38+ HLADR+ and the BRIEF or CBCL. Kapetanovic et al. reported a positive association between both CD4+ CD38+ HLADR+ and CD4+ CD38− HLADR+ cells and neurodevelopmental outcomes as measured with the full-scale IQ (FSIQ) from Bayley scales of infant development (BSID-II) and Wechsler test and a negative association on the same test with CD4+ CD38+ HLADR− cells, suggesting that CD4+ cell activation status could play a role in the development of neurocognitive deficits (Kapetanovic et al. Reference Kapetanovic, Aaron, Montepiedra, Burchett and Kovacs2012).
Limitations
Our results must be viewed in light of several limitations. First, results are based on cross-sectional data and therefore we cannot state causality of the observed associations. However, our results are supported by previous studies with similar findings (Jeremy et al. Reference Jeremy, Kim, Nozyce, Nachman, McIntosh, Pelton and Stanley2005; Mekmullica et al. Reference Mekmullica, Brouwers, Charurat, Paul, Shearer, Mendez and Smith2009).
In our bivariate and multiple logistic regression models, we were unable to explore and adjust for sociodemographic (e.g. delinquent behavior, age, school competence, orphans) and economic factors (e.g. low income) that have been reported to impact on the association between HIV and behavioral and emotional factors as measured by the CBCL and BRIEF (Bomba et al. Reference Bomba, Nacinovich, Oggiano, Cassani, Baushi, Bertulli and Badolato2010; Tadesse et al. Reference Tadesse, Berhane Tsehay, Girma Belaineh and Alemu2012; Mellins & Malee, Reference Mellins and Malee2013; Llorente et al. Reference Llorente, Brouwers, Leighty, Malee, Smith, Harris and Chase2014).
Although standardized norms for these neuropsychological measures are not available for Uganda for the BRIEF, the standardized scores are only used for research purposes within the study sample to assess the correspondence between immunological factors and child measures internally.
Given that neurobehavioral assessment was the primary goal of the randomized control trial, limited markers of T-cell activation were collected, limiting our capacity to compare between different T-cell subsets or to include additional markers in the analysis. Although a wider range of T-cell activation markers would have provided additional information regarding the role that immune cell activation has on neurobehavioral outcomes of aging children living with HIV, we believe the results presented here add to the role that commonly used T-cell activation markers have as predictors of behavioral problems within this group.
Finally, this analysis was limited to the assessment of limited individual sociodemographic, clinical and immunological factors as correlates of neurobehavioral outcomes. Future research should aim to assess economic, environmental and social factors (Gadow et al. Reference Gadow, Angelidou, Chernoff, Williams, Heston, Hodge and Nachman2012) that could impact behavior among children living with HIV, as well as a detailed assessment of factors related to ART (e.g. time of initiation and adherence) that could also modify behavioral outcomes (Laughton et al. Reference Laughton, Cornell, Boivin and Van Rie2013; Naar-King et al. Reference Naar-King, Montepiedra, Garvie, Kammerer, Malee, Sirois and Nichols2013).
Conclusion
Identifying HIV-1 infected children who are at greatest risk of poorer behavioral outcomes is critical for timely and optimal therapeutic and preventive care. Viral load appears to be a plausible virological marker given that it is now being routinely collected in resource constrained settings.
Given that neurodevelopmental problems among children living with HIV are likely to persist as they move onto adolescence and adulthood, it is important to identify behavioral problems within this group and to provide access to comprehensive psychosocial care and support aiming to prevent risky sexual behavior, substance use and to promote ART adherence (Laughton et al. Reference Laughton, Cornell, Boivin and Van Rie2013; Mellins & Malee, Reference Mellins and Malee2013).
Acknowledgements
This work was funded by the National Institutes of Health (MJB., BG, PB, NN, RO grant number R34 MH084782); a University of Michigan (U-M) Global Reach Faculty Mentored Structured Overseas Project (BG, MJB); and a U-M Global Reach Faculty Development Award (BG, MJB). The study activities on site were conducted by members of the Global Health Uganda pediatric HIV study team in Kayunga, Uganda. Team members were Sylvia Malemo Elyanu, Janet Nafula Omita, Betty Nyangoma, Peter Bazira, Godfrey Nyanja Musoke, Agatha Kuteesa, Mohammed Ssemogerere and Madina Muteesi. Their efforts are greatly appreciated.
Financial support
R34 MH084782 provided to the authors MB, BG, NN, RO, PB.
Conflict of interest
None.





