Dietary guidelines across the world have provided consistent recommendations regarding the increased intake of fruits, vegetables or whole grains(1–Reference Wang, Lay and Yu3). Although several components of the diet may be involved in the potential health benefit, fruit, vegetables, legumes and whole grains are thought to play a role through their high concentrations of antioxidant vitamins and fibre(Reference Slavin and Lloyd4). Epidemiological studies have widely reported that a diet rich in fruit, vegetables and whole grains is associated with a reduced risk of several chronic diseases(Reference Slavin and Lloyd4–Reference Chen, Tong and Xu9). More specifically, regarding dietary fibre intake, many studies have reported the importance of its consumption for health, to prevent chronic diseases such as CVD(Reference Veronese, Solmi and Caruso10), type 2 diabetes(Reference Weickert and Pfeiffer11) and some cancers(12).
Regarding lung health, dietary fibre intake has been associated with a lower risk of respiratory disease mortality(Reference Jacobs, Andersen and Blomhoff13), a greater lung function(Reference Jacobs, Andersen and Blomhoff13, Reference Hanson, Lyden and Rennard14) and a decreased risk of chronic obstructive pulmonary disease (COPD)(Reference Kaluza, Harris and Wallin15, Reference Varraso, Willett and Camargo16) and COPD symptoms(Reference Butler, Koh and Lee17). Diets low in dietary fibre have been shown to affect the immune system and are thought to promote the development of a variety of immune disorders, including allergies and asthma(Reference McKenzie, Tan and Macia18). To our knowledge, two studies have been conducted to evaluate the association between dietary fibre and asthma in adults(Reference Berthon, Macdonald-Wicks and Gibson19, Reference Halnes, Baines and Berthon20). Berthon et al. reported an inverse association between total fibre intake and severe persistent asthma(Reference Berthon, Macdonald-Wicks and Gibson19), and Halnes et al. an inverse association between soluble fibre intake and airway inflammation in asthma(Reference Halnes, Baines and Berthon20). Both studies were performed among asthmatics only and without investigating different types nor sources of fibre.
Asthma is a heterogeneous disease with different phenotypes, variable clinical manifestations and temporal phenotypic variability(Reference Boudier, Curjuric and Basagaña21). Thus, rather than dichotomous definition of asthma, such as doctor-diagnosed asthma, in epidemiological studies, it is necessary to be able to capture the complexity of asthma. The asthma symptom score has been recommended for the study of risk factors in asthma(Reference Sunyer, Pekkanen and Garcia-Esteban22, Reference Pekkanen23) and is particularly relevant when investigating association with diet, as it can be used for both participants with and without asthma allowing integration of different asthma phenotypes and different levels of asthma prevention. Likewise, achieving and maintaining a good asthma control is the main goal of asthma management, and better understanding as to why asthma is getting worse (or better) in a substantial proportion of patients would be very informative. To our knowledge, no previous studies have been conducted to investigate the potential role of different types and sources of dietary fibre both on asthma symptoms and on asthma control.
The objectives of the present study conducted in a large cohort of French adults were to (1) investigate the cross-sectional association of total dietary fibre, soluble and insoluble fibre and different sources of fibre (cereals, vegetables, fruit, seeds and legumes) with the asthma symptom score and (2) further evaluate the impact of dietary fibre consumption on asthma control.
Methods
Population
Subjects were participants of the NutriNet-Santé Study, an ongoing web-based prospective observational French cohort of volunteers aged 18 years and older, launched in May 2009. Briefly, its main objective is to investigate the relationship between nutrition and health as well as of determinants of dietary behaviours and nutritional status. At enrolment, participants complete a baseline set of questionnaires; and during the follow-up, they receive monthly automated emails informing that new questionnaires are available in the secured platform. More detailed information has been published elsewhere(Reference Hercberg, Castetbon and Czernichow24). The NutriNet-Santé study is conducted according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the French Institute for Health and Medical Research (IRB Inserm no. 0000388FWA00005831) and the ‘Commission Nationale de l’Informatique et des Libertés’ (CNIL no. 908450/no. 909216). It is registered at clinicaltrials.gov as NCT03335644. All participants provided electronic informed consent.
Dietary data collection
Participants were invited to complete three non-consecutive self-administered web-based 24-h dietary records randomly distributed within a 2-week period at baseline and every 6 months. Self-administered web-based 24-h dietary records have been validated against urinary(Reference Lassale, Castetbon and Laporte25) and plasma biomarkers(Reference Lassale, Castetbon and Laporte26) and against interview by a trained dietitian(Reference Touvier, Kesse-Guyot and Méjean27).
Participants reported all foods and beverages at each eating occasions during the day. Portion size for each foods and beverages were directly entered as grams or volumes or estimated using previously validated photographs(Reference Le Moullec, Deheeger and Preziosi28). Daily dietary intake was calculated as the average of at least three 24-h dietary records. Energy underreporting participants were identified and excluded using the validated method developed by Black(Reference Black29). Black’s equations are based on an estimate of the person’s BMR calculated via Schofield’s equations(Reference Schofield30) and taking into account sex, age, height and weight as well as the physical activity level (PAL), number of 24-h records, intra-individual variabilities of reported energy intake and BMR and intra-/inter-variabilities of PAL.
Dietary fibre intake was calculated in g/d, and we considered total dietary fibre, soluble and insoluble fibres (types of fibre) and cereal fibre, vegetable fibre, fruit fibre, seed fibre and legume fibre (sources of fibre). We obtained the dietary fibre content of each food from the NutriNet-Santé food composition table(31), which is a compilation of data from the Supplementation en Vitamines et Minéraux AntioXydants (SU.VI.MAX) study food database, published literature and other food composition tables from different European countries. For mixed dishes, fibre contents were calculated using nutrient contents of individual foodstuffs and usual recipes. Dietary fibre intake (total, types and sources) was adjusted for total energy intake using the residual method(Reference Willett, Howe and Kushi32) and was classified based on sex-specific quintiles.
To assess the overall nutritional quality of the diet, we computed the modified Programme National Nutrition Santé-guidelines score (mPNNS-GS), without physical activity, reflecting adherence to the French nutritional recommendations. The mPNNS-GS includes twelve components: eight components referred to food-serving adequacy and four referred to moderation in consumption. Moreover, points are deducted for overconsumption of salt, added sugars or when energy intake exceeds the estimated energy needs by more than 5 %. Scoring has been described in detail elsewhere(Reference Estaquio, Kesse-Guyot and Deschamps33), and the final score ranges from (possible) negative scores to 13·5.
The respiratory survey in the NutriNet-santé cohort
To improve the respiratory characterisation of the participants of the cohort, a non-mandatory detailed questionnaire on respiratory health based on international standardised recommendations(Reference Burney, Luczynska and Chinn34) was proposed in April 2016 to 121 568 active participants included in the cohort at this date. As of June 2017, the survey was filled in by 40 152 adults. Respondents (n 40 152) were significantly older as compared with non-respondents (n 81 416). After adjustment for age, they were also more likely never smokers, had a higher educational level, were more physically active and less likely to be obese(Reference Andrianasolo, Kesse-Guyot and Adjibade35). Ever asthma was defined by at least one positive answer to the question ‘Have you ever had asthma?’ in main questionnaires or by a positive answer to ‘Have you ever had an asthma attack?’ or ‘Have you ever had an attack of shortness of breath at rest with wheezing’ in the respiratory survey.
We used the asthma symptom score(Reference Sunyer, Pekkanen and Garcia-Esteban22, Reference Pekkanen23) which has been proposed previously as a continuous measure of asthma in epidemiological studies. It is a validated score, ranging from 0 to 5, based on the number of respiratory symptoms during the past 12 months.
The asthma control test (ACT)(Reference Schatz, Sorkness and Li36) was used to evaluate asthma control. It is a validated self-administered questionnaire based on five questions on activity limitations, frequency of symptoms, frequency of use of quick-relief medication and the subjective perception of the level of asthma control during the previous 4 weeks. Each item is scored from 1 to 5, and the total ACT score ranges from 5 to 25. Participants with asthma were classified as well-controlled (ACT > 19) or uncontrolled (ACT ≤ 19)(Reference Schatz, Sorkness and Li36).
Allergic rhinitis was defined as a positive answer to ‘Have you ever had allergic rhinitis? or ‘Have you ever had hay fever?’ Information about family history of asthma was also collected (yes/no).
Assessment of covariates
At baseline, validated self-administered questionnaires were used to collect data which includes sex, age, educational level (<13, 14, 15–16, ≥17 years) and smoking status (never smokers, ex-smokers, current smokers). Among ever smokers, pack-years were calculated to estimate the amount of tobacco smoke. BMI was calculated as weight (kg)/height2 (in m2) and categorised according to the WHO classification (<18·5, 18·5–24, 25–29, ≥30 kg/m2)(37). Self-reported leisure-time physical activity was assessed using the French short form of the International Physical Activity Questionnaire(Reference Hagströmer, Oja and Sjöström38). The weekly activity-associated energy expenditure expressed in metabolic equivalent task in minutes per week was estimated, and three categories of physical activity were defined (vigorous (≥60 min/d), moderate (30–59 min/d) and low (<30 min/d)).
Statistical analysis
From the 40 152 participants who filled in the respiratory survey, we included those with at least three dietary records from baseline till their 2 years of follow-up, that is, mean = 8·0 (sd 3·0), and who were not identified as energy underreporters to account for intra-individual variation in intake and estimate usual intake. Our analytic population included 26 640 women and 8740 men (online Supplementary Fig. S1). Regarding asthma control, 2094 women and 557 men completed the ACT questionnaire out of the 3116 participants who reported ever asthma.
All analyses were conducted separately among women and men since the effect of dietary fibre may differ in women and men(Reference Fernstrand, Bury and Garssen39). As previously done for this score, association between quintiles of total fibre, types and sources with the asthma symptom score (treated as a continuous variable) were evaluated using a binomial negative regression models(Reference Jacquemin, Sunyer and Forsberg40). Multivariable models were adjusted for age, smoking status, pack-years in smokers, educational level, leisure-time physical activity, total daily energy intake, allergic rhinitis and family history of asthma. Tests for linear trend were performed using median value for each quintiles of fibre intake as a continuous variable in the model.
We performed several sensitivity analyses. First, we tested whether the association between dietary fibre intake and the asthma symptom score was modified by smoking status, and we formally tested the interaction between fibre intake and smoking. Second, since growing evidence suggests that dietary fibre is associated with overweight and obesity in adults(Reference Anderson, Baird and Davis41), and BMI a likely potential mediator in the diet–asthma association(Reference Bédard, Dumas and Kauffmann42), we performed further analysis stratified by BMI and tested the interaction between fibre intake and BMI. Then since a high fibre intake might reflect an overall healthy diet, and to account for the overall remaining effect of diet in the fibre–asthma association, we performed a sensitivity analysis by adjusting for overall nutritional quality of diet assessed by the mPNNS-GS, a score reflecting adherence to French nutritional recommendations(Reference Estaquio, Kesse-Guyot and Deschamps33). We also performed analysis stratified across mPNNS-GS sex-specific tertiles. Finally, we performed a sensitivity analysis among participants who never reported asthma from their inclusion in the NutriNet-Santé cohort till their answer to the respiratory survey (a likely free of asthma population).
For asthma control, association with quintiles of dietary fibre (total, types and sources) was evaluated using logistic regression. Main models were adjusted for age, smoking, pack-years, educational level, leisure-time physical activity and total daily energy intake. Further analysis was also performed by adjusting for the overall nutritional quality of diet assessed by the mPNNS-GS.
Missing data for covariates were handled by multiple imputations (n 10), according to a Markov chain Monte Carlo approach(Reference Yuan43).
We used SAS software version 9.4 (SAS Institute) to analyse the data. All the tests were two sided and the significance level was set at 0·05.
Results
The descriptive characteristics of the participants according to quintiles of energy-adjusted total dietary fibre are shown in Table 1. The mean age was 53(Reference Hanson, Lyden and Rennard14) years in women and 59(Reference Jacobs, Andersen and Blomhoff13) years in men. The average dietary fibre intake was 20·3 g/d among women and 20·5 g/d among men. Main contributors to total fibre intake were cereals (29 % in women and 31 % in men), vegetables (26 % in women and 25 % in men), fruits (20 % both in women and men), legumes (3 % in women and 4 % in men) and nuts (2 % both in women and men). Total dietary fibre, soluble and insoluble fibre intakes were highly correlated with dietary fibre intake from fruit and legumes both among women (online Supplementary Table S1) and men (online Supplementary Table S2). Among women and men, participants in the highest quintile of total dietary fibre were significantly older, less likely to be current smokers, more physically active during leisure time, had a higher educational level and were less likely to be obese than participants in the lowest quintile of total dietary fibre.
Table 1. Characteristics of the participants, before imputation, according to the quintiles of total fibre intake, among women (n 26 640) and men (n 8740) from the NutriNet-Santé study
(Mean values and standard deviations; numbers and percentages)
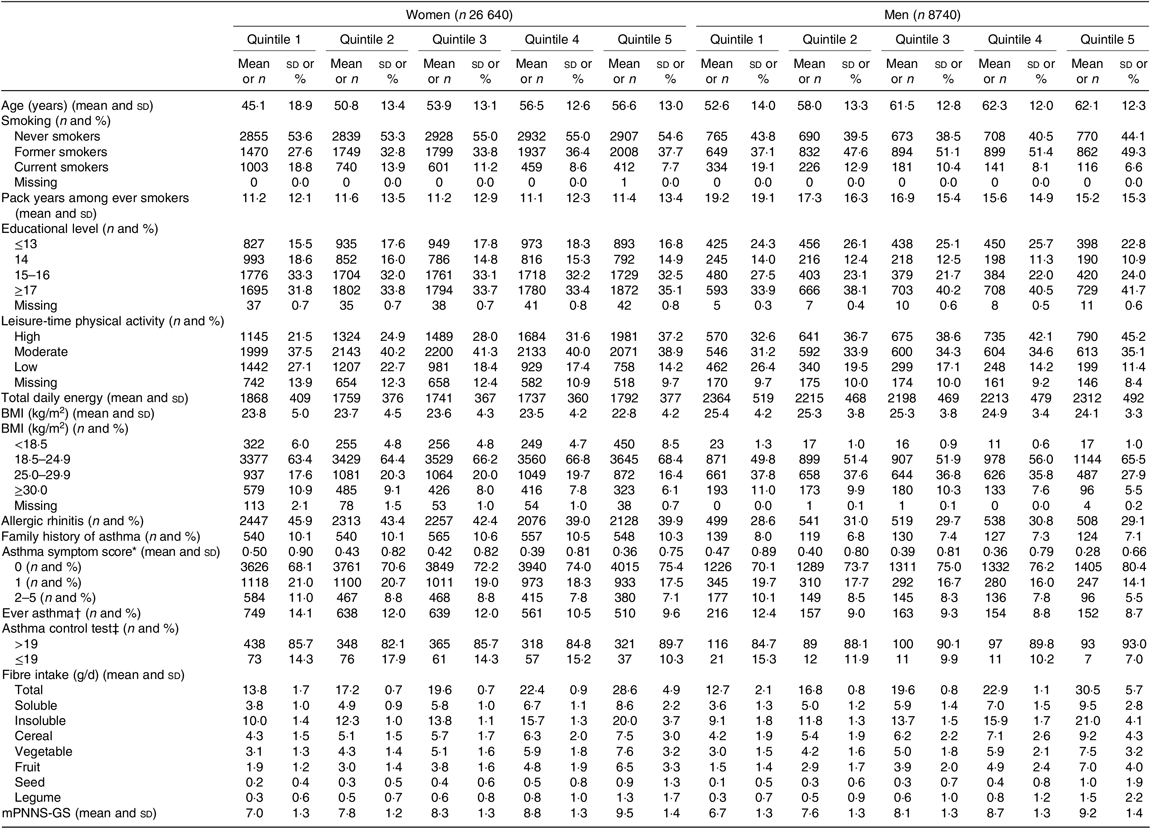
mPNNS-GS, Modified Programme National Nutrition Santé guidelines score.
* Number of respiratory symptoms during the past 12 months: (1) breathless while wheezing, (2) woken up with chest tightness, (3) attack of shortness of breath at rest, (4) attack of shortness of breath after exercise and (5) woken by attack of shortness of breath. Each item is scored from 0 to 1, and the total asthma symptom score ranges from 0 to 5.
† Defined by at least one positive answer to the question ‘Have you ever had an asthma attack?’ in main questionnaire (baseline or follow-up), and by a positive answer to ‘Have you ever had an asthma attack?’ or ‘Have you ever had an attack of shortness of breath at rest with wheezing’ in the respiratory survey (2016).
‡ Among 2063 women and 546 men. Based on five questions in the last 4 weeks on: (1) activity limitations: asthma keep from getting as much done at work, school or at home, ‘some of the time’ to ‘all of the time’; (2) shortness of breath: ‘3–6 times/week’ to ‘more than once daily’; (3) woken up by asthma symptoms at night: ‘once/week’ to ‘every night’; (4) use β-agonist inhaler ‘2 times/week’ to ‘3+ times daily’ and (5) self-rated asthma control: ‘somewhat controlled’ to ‘not controlled at all’. Each item is scored from 1 to 5, and the total asthma control test score ranges from 5 to 25.
Types of dietary fibre and asthma symptom score
In our study, 28 % of women and 25 % of men reported at least one asthma symptom. After adjustment for potential confounders, total dietary fibre intake was significantly negatively associated with asthma symptoms among both women and men (Fig. 1). The OR for the participants in the highest quintile of total dietary fibre was 0·73 (95 % CI 0·67, 0·79) in women (Table 2) and 0·63 (95 % CI 0·55, 0·73) in men (Table 3) compared with participants in the lowest quintile of total dietary fibre intake, with a significant trend (P < 0·0001). Further adjustment for the mPNNS-GS did not modify the association (online Supplementary Tables S3 and S4).
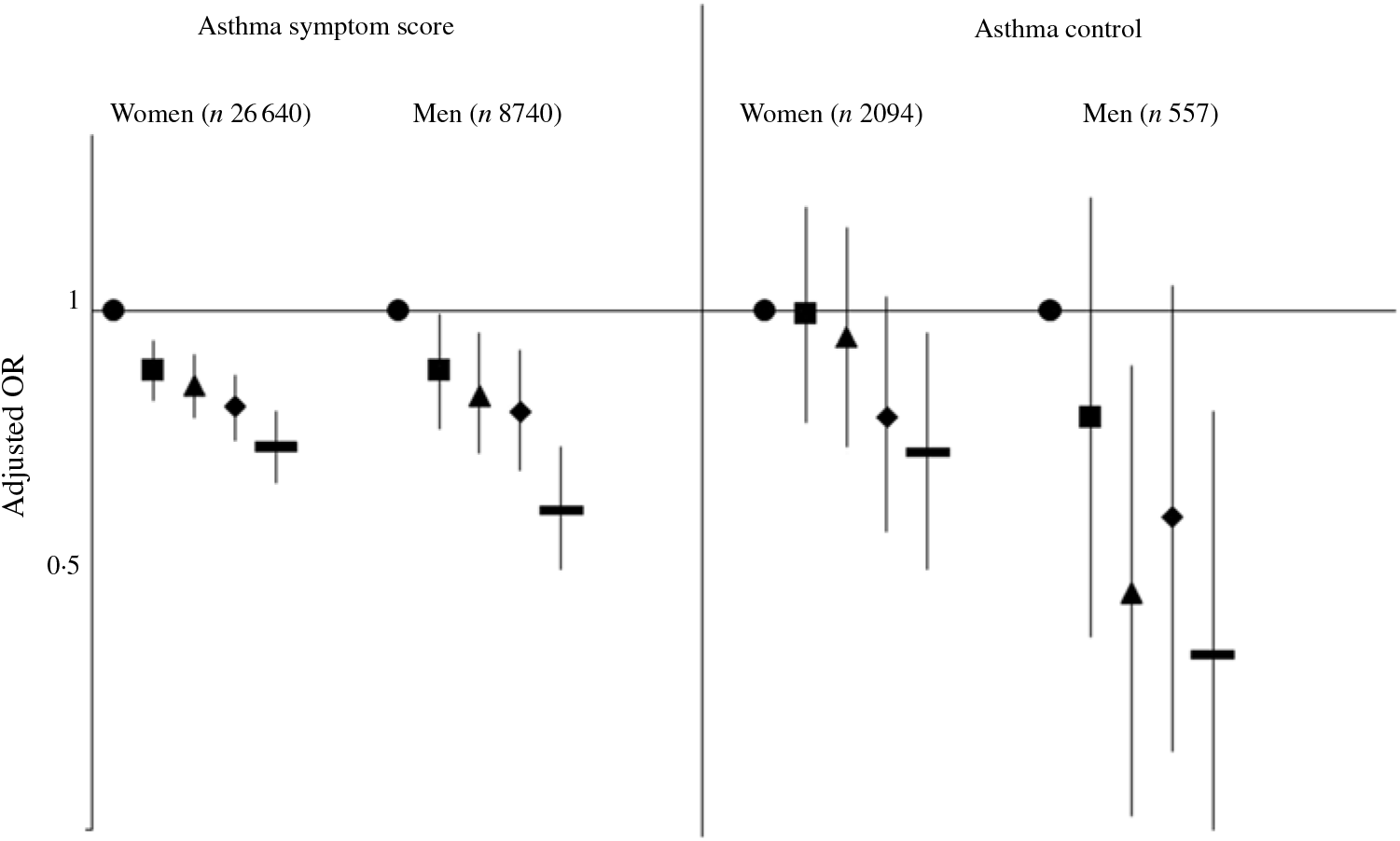
Fig. 1. Associations between quintiles of total dietary fibre intake with the asthma symptom score (continuous) and the asthma control (controlled v. uncontrolled) among women and men. Values are adjusted odds ratios and 95 % confidence intervals.
Table 2. Association between quintiles of dietary fibre intake and the asthma symptom score (continuous variable) among women from the NutriNet-Santé study (n 26 640)
(Odds ratios and 95 % confidence intervals)
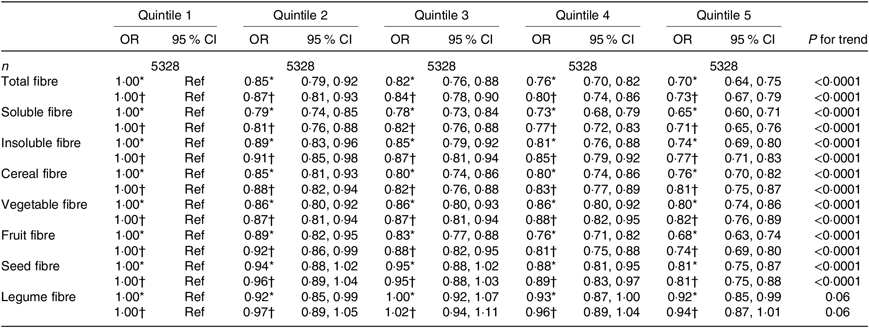
Ref, reference.
* Adjusted for age.
† Further adjusted for smoking, pack-years (among ever smokers), educational level, leisure-time physical activity, total daily energy, allergic rhinitis and family history of asthma.
Table 3. Associations between quintiles of dietary fibre intake and the asthma symptom score (continuous variable) among men from the NutriNet-Santé study (n 8740)
(Odds ratios and 95 % confidence intervals)
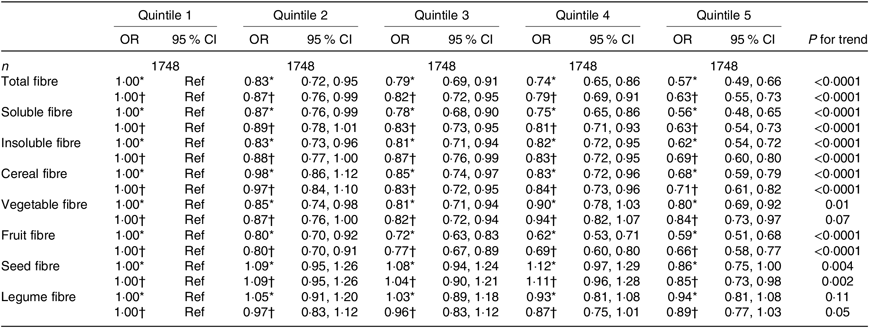
Ref, reference.
* Adjusted for age.
† Further adjusted for smoking, pack-years (among ever smokers), educational level, leisure-time physical activity, total daily energy, allergic rhinitis and family history of asthma.
After stratification on smoking habits, BMI and the mPNNS-GS, we still reported OR lower than 1 in each group (Fig. 2). For smoking, we observed association of similar magnitude in never, former and current smokers, especially among women (Fig. 2). The interaction between total dietary fibre intake with smoking status was not statistically significant (P = 0·66 in women and 0·39 in men). For BMI, the association between total dietary fibre intake and asthma symptom was significant only among participants with BMI < 30·0 kg/m2, but we reported no significant interaction between total dietary fibre and BMI (P = 0·13 in women and 0·36 in men). For the mPNNS-GS, we observed similar association between total dietary fibre intake and asthma symptom in each tertile, with no significant interaction between mPNNS-GS and total dietary fibre (P = 0·19 in women and 0·29 in men). Finally, when analyses were restricted to participants without ever asthma (n 24 159 women and 8069 men), we found similar associations between dietary fibre intake and the asthma symptom score: OR for the participants in the highest quintile of total dietary fibre was 0·74 (95 % CI 0·68, 0·81) in women and 0·68 (95 % CI 0·58, 0·79) in men, as compared with participants in the lowest quintile.
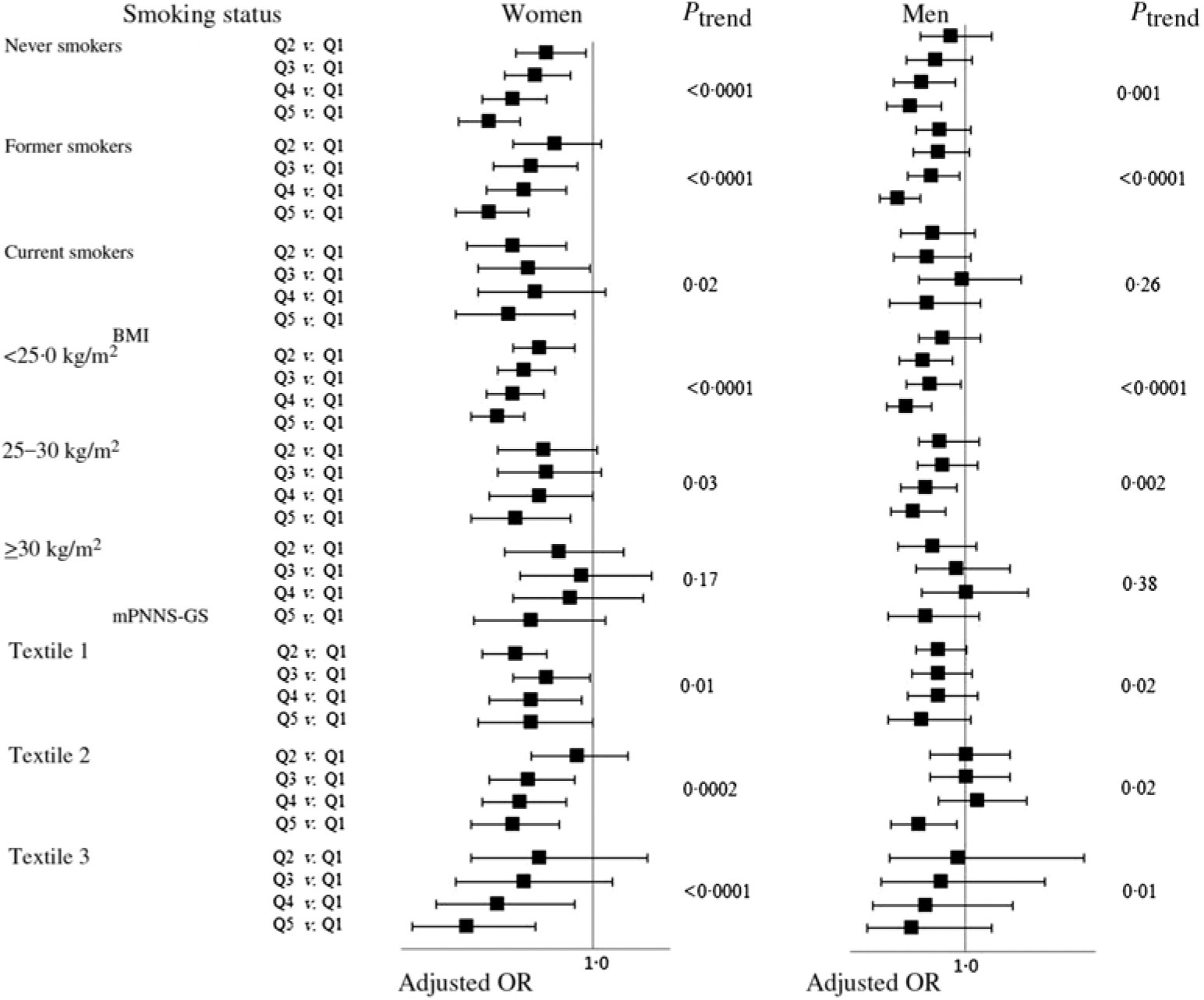
Fig. 2. Associations between quintiles (Q) of total dietary fibre intake and the asthma symptom score (continuous variable) among women and men from the NutriNet-Santé study, stratified according to smoking, BMI and modified Programme National Nutrition Santé-guidelines score (mPNNS-GS). Models were adjusted for age, smoking (when appropriated), pack-years (among ever smokers), educational level, leisure-time physical activity, total daily energy, allergic rhinitis and family history of asthma. Values are adjusted odds ratios and 95 % confidence intervals.
When looking at the type of fibre (soluble or insoluble), we observed a significant inverse association of similar magnitude with asthma symptoms in both women and men (Tables 2 and 3).
Sources of dietary fibre and asthma symptom score
After adjustment for potential confounders, we observed a significant inverse association between cereal fibre, fruit fibre and seed fibre with asthma symptoms, both among women (Table 2) and men (Table 3), with OR for the highest quintile compared with the lowest quintile ranging from 0·66 to 0·85. In women, after adjustment for potential confounders, vegetable fibre was also associated with lower asthma symptoms (OR for the highest quintile compared with the lowest quintile was 0·82 (95 % CI 0·76, 0·89)). Among men, the association was borderline significant for legume fibre (OR for the highest quintile compared with the lowest quintile was 0·89 (95 % CI 0·77, 1·03), with a significant trend (P for trend = 0·05). Overall, further adjustment for the mPNNS-GS did not substantially modify the association among both women and men (online Supplementary Tables S3 and S4).
Types of dietary fibre and asthma control
We observed a significant inverse association between higher dietary fibre intake and uncontrolled asthma, both among women and men (Fig. 2). After adjustments for potential confounders, OR for participants in the highest quintile was 0·72 (95 % CI 0·55, 0·95) in women (Table 4) and 0·45 (95 % CI 0·26, 0·79) in men (Table 5) compared with participants in the lowest quintile of total dietary fibre intake. Further adjustment for the mPNNS-GS did not modify the results (online Supplementary Table S5 and S6).
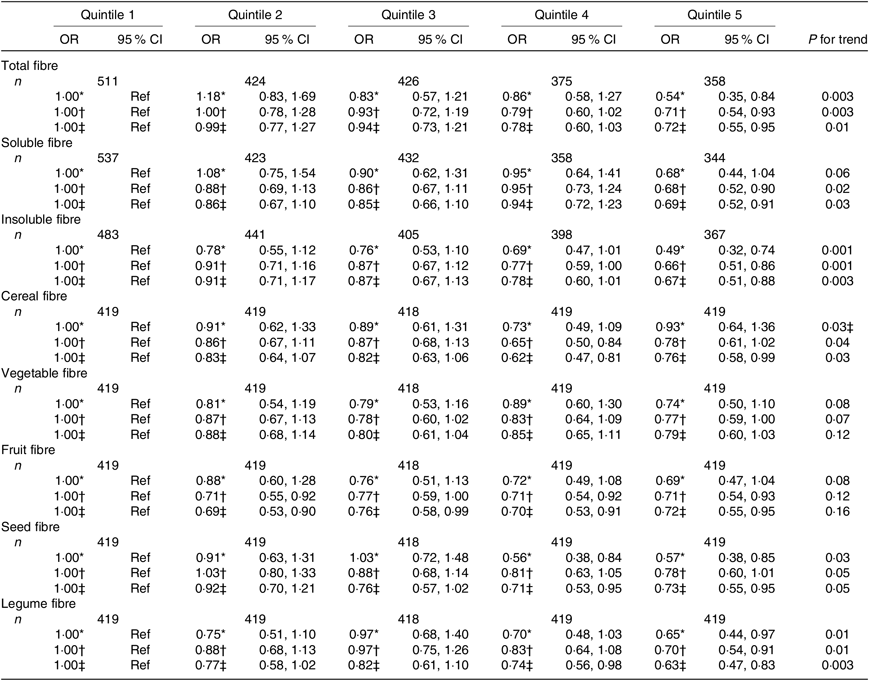
Table 4. Association between quintiles of dietary fibre intake and the asthma control test score among women from the NutriNet-Santé study (n 2094)
(Odds ratios and 95 % confidence intervals)
Ref, reference.
* Adjusted for age.
† Further adjusted for smoking and pack-years (among ever smokers).
‡ Further adjusted for educational level, leisure-time physical activity, total daily energy.
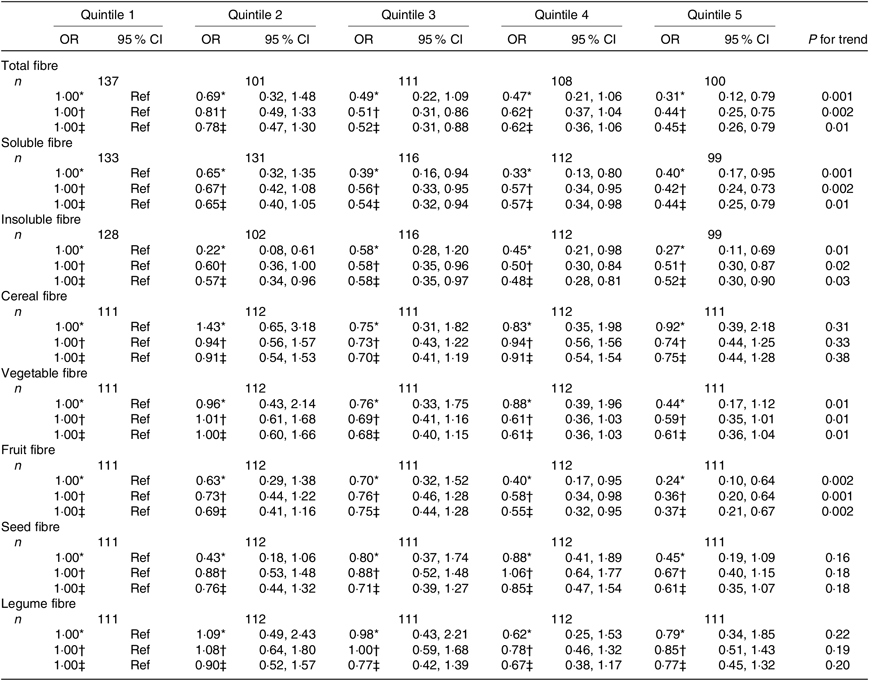
Table 5. Associations between quintiles of dietary fibre intake and the asthma control test score among men from the NutriNet-Santé study (n 557)
(Odds ratios and 95 % confidence intervals)
Ref, reference.
* Adjusted for age.
† Further adjusted for smoking and pack-years (among ever smokers).
‡ Further adjusted for educational level, leisure-time physical activity, total daily energy.
Regarding the fibre types, higher intake of both soluble and insoluble fibres was also significantly inversely associated with uncontrolled asthma in women (Table 4) and men (Table 5).
Sources of dietary fibre and asthma control
In women, after adjustment for confounders, we observed that greater intake of cereal, seed and legume fibre was significantly negatively associated with uncontrolled asthma (Table 4). In men, we observed significantly inverse association for vegetable and fruit fibre intake (Table 5). Further adjustment for the overall quality of the diet did not substantially change the results except for the legume fibre intake in women for which the association was no longer statistically significant (online Supplementary Tables S5 and S6).
Discussion
In this large study of French adults, we observed that higher dietary fibre intake (total, regardless of the type, namely, soluble and insoluble), was associated with less asthma symptoms in both women and men. Association was consistent in several sub-populations and after adjustment for several potential confounders. When specific sources of fibre were taken into consideration, intake of fibre from cereals, fruit and seeds was most consistently associated with less asthma symptoms. We also observed inverse association between uncontrolled asthma and greater intake of dietary fibre, mainly for total dietary fibre, soluble and insoluble fibres and fibre intake from cereals, fruit, seeds and legumes.
To our knowledge, this is the first study to investigate the association of both asthma symptoms and asthma control with total dietary fibre and types (soluble and insoluble) of fibre. Consistent with our findings, Berthon et al.(Reference Berthon, Macdonald-Wicks and Gibson19) showed that asthmatics with severe disease have an altered pattern of dietary intake with reduced total fibre intake, which was related to worsened lung function and airway inflammation, as compared with healthy controls. Likewise, the recent randomised controlled trial study conducted by Halnes et al.(Reference Halnes, Baines and Berthon20) investigates the effect of a single dose of soluble fibre on airway inflammation biomarkers in stable asthma and reported a beneficial role of soluble fibre to reduce airway inflammation. Dietary fibre can be subcategorised by its solubility into water-insoluble and water-soluble fibres(Reference Dhingra, Michael and Rajput44); and although differences between these two types are mostly chemical and physical, and to a lesser extent physiological(Reference Dikeman and Fahey45), it is relevant to evaluate their separate effect on asthma. Our results extended previous findings regarding the role of soluble fibre and also suggested the role of insoluble fibre for both asthma symptoms and asthma control.
Overall, our findings regarding the role of specific sources of dietary fibre are in line with previous published studies showing that a healthy diet rich in fruits, vegetables or whole-grain cereals, and thus potentially rich in dietary fibre, may have beneficial role against asthma symptoms and asthma control(Reference Ma, Strub and Lv46-Reference Li, Kesse-Guyot and Dumas48). However, those studies have not focused on fibre per se, and therefore, its effect might have been combined or diluted with other constituents of the diet with anti- or proinflammatory/antioxidant properties. Regarding associations between sources of fibre with various health outcomes such as CVD or COPD, significant results were mostly reported for cereal fibre(Reference Kaluza, Harris and Wallin15, Reference Varraso, Willett and Camargo16, Reference Cho, Qi and Fahey49). In our study, besides cereal fibre, we also reported consistent significant associations between asthma symptoms and asthma control with various sources of dietary fibre including fruits and vegetables.
Asthma is a chronic inflammatory disease and several potential mechanisms underlying the dietary fibre–asthma association have been proposed, including inflammation and more recently, an imbalance in the gut microbiome. Regarding inflammation, several epidemiological studies have reported that a greater intake of total fibre was associated with lower level of C-reactive protein (CRP)(Reference Ma, Griffith and Chasan-Taber50) and various proinflammatory cytokines such as lower plasma levels of TNF-α receptor 2 and IL-6(Reference Ma, Hébert and Li51). Consistently, a recent randomised controlled trial conducted by Honsek et al. reported a decrease in CRP level after 1 year intervention with insoluble fibre supplementation or promoting healthy diet (including among others, an increase in fibre intake)(Reference Honsek, Kabisch and Kemper52). In addition, in the recent pilot study by Halnes et al.(Reference Halnes, Baines and Berthon20), when comparing participants with stable asthma to the control group, a higher consumption of soluble fibre was associated with lower levels of sputum neutrophils, macrophages and lymphocytes. However, since we also found significant association with insoluble fibre, our results may suggest that other mechanisms are likely to be involved. Likewise, a meta-analysis by Aune et al. reported a significant beneficial effects of diets rich in whole grain and high in insoluble fibre and also several health outcomes including respiratory inflammatory diseases(Reference Aune, Keum and Giovannucci53). Regarding microbiota, a growing number of investigators hypothesise that an imbalance in the gut microbiome caused by changes in the diet over the past decades may cause dysfunction of the immune system, leading to the development of asthma and allergy(Reference McKenzie, Tan and Macia18). Microbiota metabolise dietary soluble fibres through fermentation and decomposition and provide SCFA(Reference Ríos-Covián, Ruas-Madiedo and Margolles54). In children, the specific gut microbiota accompanied by a reduction of SCFA was associated with a higher risk of incident asthma(Reference Arrieta, Stiemsma and Dimitriu55), and it has been shown that fibre intake altered the composition of both the gut and, to a lesser degree the lung, microbiota(Reference Trompette, Gollwitzer and Yadava56). In this line, high dietary fibre consumption might be relevant for primary and secondary prevention.
The present study has few limitations that should be considered. First, caution is needed when extrapolating our results since participants from the NutriNet-Santé cohort are all volunteers involved in a long-term cohort focused on nutrition and health and were overall more health conscious and interested in nutritional issue, with higher educational level and socio-professional status(Reference Andreeva, Deschamps and Salanave57). Besides, proportion of men is low, which might have resulted in a relative lack of statistical power, especially when assessing the association between fibre intake and asthma control among men. Second, although dietary data were prospectively collected, the respiratory data used in our study were collected in a cross-sectional manner, limiting the conclusions that could be drawn with regard to causality. Nevertheless, the association remained significant in participants without ever asthma. Moreover, data were collected from self-reported questionnaires, which are more prone to biases and should also be interpreted with caution. However, self-reporting is widely used in epidemiological studies and, in the context of the NutriNet-Santé cohort, validation studies showed the accuracy of self-reported data(Reference Lassale, Castetbon and Laporte25, Reference Lassale, Castetbon and Laporte26, Reference Lassale, Péneau and Touvier58) along with reduced social desirability biases(Reference Kesse-Guyot, Assmann and Andreeva59). Third, given the observational design of our study, we cannot rule out the possibility of residual confounding. Fourth, potential overlap between asthma and COPD might also contribute to the association. However, it is unlikely since stratification by smoking status yielded consistent results among never smokers. Finally, high dietary fibre intake is one characteristic of a healthy diet, which was consistently reported to be associated with asthma, and, thus, other dietary components of these diets might also have contributed to the association. However, we reported similar associations when results were further adjusted for the overall nutritional quality of the diet.
Strengths of the present study include its sample size that gave us the possibility to perform stratified analyses, the availability of information about potential confounders and the fact that we could have estimated individual associations between asthma with total fibre, soluble and insoluble fibres or different sources of fibre. In addition, we used validated tools to assess asthma symptoms and asthma control. Finally, to better reflect usual intake, we used repeated 24-h dietary records over a long period to assess dietary data.
In conclusion, we observed that higher dietary fibre intake was associated with fewer asthma symptoms and greater asthma control. Overall, our results suggested that dietary fibre might play an important role in the prevention of asthma and its lack of control and thus reinforce the growing evidence of benefit of dietary fibre for lung health. Furthermore, these findings lent further support for public health nutrition policies and practices that encourage healthy eating behaviours with an increased consumption of dietary fibre, mostly for insoluble fibres or fibres from cereals.
Acknowledgements
The authors thank Younes Esseddik, Thi Hong Van Duong, Paul Flanzy, Régis Gatibelza, Jagatjit Mohinder (computer scientists), Cédric Agaësse (dietitian), Julien Allègre, Nathalie Arnault, Laurent Bourhis and Fabien Szabo de Edelenyi, PhD (data-managers/biostatisticians) and Fatoumata Diallo, MD (physician). We thank all the volunteers of the NutriNet-Santé cohort.
The NutriNet-Santé Study was supported by the following public institutions: Ministère de la Santé, Santé Publique France, Institut National de la Santé et de la Recherche Médicale, Institut National de la Recherche Agronomique, Conservatoire National des Arts et Métiers and Université Paris 13.
Author contributions were as follows. R. M. A., P. G. and R. V. designed and conducted the research; E. K. G., N. D. P., M. T., S. H., P. G. and R. V. provided essential reagents or provided essential materials; R. M. A., and R. V. analysed data or performed statistical analysis; R. M. A., P. G. and R. V. wrote the manuscript and had primary responsibility for the final content; R. M. A., S. H., E. K. G., N. D. P., M. T., P. G. and R. V. were involved in interpreting the results and editing the manuscript for important intellectual content. All authors read, edited and approved the final manuscript.
The authors have no conflicts of interest to declare.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114519001843










