Introduction
Maintaining the blood:milk barrier is one of the most important factors for galactopoiesis. Mammary epithelium integrity is reflected by lactose in blood, since the only way for lactose to appear in blood is leakage across the mammary epithelium. Mammary tight junctions become leaky when milk accumulates in the udder (Stelwagen et al., Reference Stelwagen, Davis, Farr, Eichler and Politis1994, Reference Stelwagen, Farr, McFadden, Prosser and Davis1997) and this is reflected by lactose in plasma which is efficiently excreted in urine. Plasma half-life for lactose has been shown to be 44 min in dairy cows (Stelwagen et al., Reference Stelwagen, Farr, McFadden, Prosser and Davis1997). Schultz et al. (Reference Schultz, Hanisch, Dumke, Springer and Beck1998) found urinary lactose <0·5 g/l in cows in established lactation with good udder health while values over 4 g/l were seen at parturition, at the beginning of an acute mastitis and around day three after drying off (Schultz et al. Reference Schultz, Hanisch, Dumke, Springer and Beck1998). Plasma lactose increased within 18 and 21 h of milk accumulation in the udder in cows and goats respectively (Stelwagen et al., Reference Stelwagen, Davis, Farr, Eichler and Politis1994, Reference Stelwagen, Farr, McFadden, Prosser and Davis1997) and returned to normal levels soon after milking, indicating a quick closure of the tight junctions (Stelwagen et al., Reference Stelwagen, Farr, McFadden, Prosser and Davis1997).
Pregnancy accelerates the post-peak lactation decline in milk yield (Salama et al., Reference Salama, Caja, Such, Casals and Albanell2005) through influence of placental hormones, most likely estrogen (Bachman et al., Reference Bachman, Hayen, Morse and Wilcox1988). Plasma estradiol increases in late pregnancy and reaches a maximum a few days before parturition. In goats, the decrease in milk yield by pregnancy is seen two months after mating. On the other hand, non-pregnant goats may continue to lactate without a significant decline in milk yield for 2–4 years (Salama et al., Reference Salama, Caja, Such, Casals and Albanell2005). Exogenous 17β-estradiol results in decreased milk yield and accelerated mammary involution in cows in mid lactation (Delbecchi et al., Reference Delbecchi, Miller, Prud'homme, Petitclerc, Wagner and Lacasse2005). Athie et al. (Reference Athie, Bachman, Head, Hayen and Wilcox1996) showed that administration of exogenous estradiol in late-lactation cows gave rise to a rapid drop in milk yield and it was suggested that estradiol induced an acceleration of normal involution with more leaky tight junctions.
The aim of this study was to investigate the effect of 17β-estradiol on mammary tight junctions in non-pregnant cows in late lactation in order to separate the effect of 17β-estradiol from other effects of pregnancy. The hypothesis was that when 17β-estradiol levels in plasma are elevated for longer than during estrus they contribute to the loss of mammary tight junction integrity and milk synthesis that often is seen in late lactation.
Materials and methods
Animals, management and experimental design
The study was performed at Kungsängen Research Centre, belonging to the Swedish University of Agricultural Sciences in Uppsala, Sweden. Five multiparous non-pregnant cows of the Swedish Red Breed weighing 561 ± 71 kg and at 289 ± 30 DIM on Day 1 of the experiment were included in the study. The study was carried out in a tie stall barn and the cows were fed individually four times daily with silage and concentrates according to Swedish standards for metabolizable energy (ME), protein and minerals based on actual milk yield and stage of gestation (Spörndly, Reference Spörndly2003). Ad libitum access to fresh water was available. Use and handling of animals was approved by the Uppsala Local Ethics Committee.
The effect of exogenous 17β-estradiol on milk synthesis, lactose in plasma and urine was investigated in the five cows. No treatment was applied during day 1–6 and 13–19. Each day between days 7 and 12, 18·2 mg 17β-estradiol was injected intramuscularly at 14·00 h. The injection solution was prepared as follows: 250 mg 17β-estradiol (Sigma) was dissolved in 25 ml Benzyl alcohol and then added to 85 ml peanut oil of injection quality (Ultrasan® vet, Pharmaxim Sweden AB, Sweden) and the liquid was gently heated and mixed until the benzyl alcohol solution was completely mixed with the oil. Blood was collected from the tail vein between 14·00 and 14·30 h, after 17β-estradiol injections, on days 1, 3, 5, 7, 8, 10, 12, 14, 15, 17 and 19 of the study. Urine was collected on the same days as the blood samples, also between 14·00 and 14·30 h.
Blood and urine samples
Blood samples were collected with evacuated tubes with sodium heparin as anti-coagulant (Venoject, Terumo Europe N.V., Leuven, Belgium). Blood samples were centrifuged (15 min at 3000 rpm) within an hour after sampling and plasma was stored at −20 °C until it was analyzed. Urine samples were obtained by stimulating the cows to urinate through stroking up to the vulva gently. Urine was collected in a bucket when the cows urinated. Aliquots of urine samples were stored at −20 °C until being analyzed. The concentration of lactose in plasma and urine was determined enzymatically (Lactose/D-galactose kit Boehringer Mannheim/R-Biopharm). The concentration of 17β-estradiol in plasma was analyzed after ether extraction using a human radioimmunoassay validated for cattle (Coat-a-Count® Estradiol Siemens Healthcare Diagnostics, Eschborn, Germany). The concentration of creatinine in urine was analyzed by use of a Technicon Auto Analyzer II (Technicon method number SE40011FH4, Technicon, Solna, Sweden). The lactose losses in urine were calculated assuming a daily urinary excretion of 29 mg creatinine/kg BW (Valadares et al., Reference Valadares, Broderick, Valadares Filho and Clayton1999).
Milk samples
The cows were machine milked twice daily at about 06·30 and 15·30. Milk yield was recorded and milk samples were collected at every milking. Milk yield recordings and milk samples were obtained with the TruTester technique (TruTest, The Netherlands). Evening milking on day 4, 11 and 18 and morning milking on day 5, 12 and 19 was performed with a milking machine that separates milk from each udder quarter (provided by DeLaval International AB, Tumba, Sweden). Cups were removed for each quarter individually when flow was <300 g/min. The weight of milk obtained from each quarter was recorded. Milk samples were collected from each quarter at these milkings. All milk samples were analyzed for fat, protein and lactose using Milko Scan FT 120 (FOSS Analytical, Hillerød, Denmark). The somatic cell count was determined by Fossomatic 5000 (FOSS Analytical, Hillerød, Denmark). The quarter milk samples obtained on day 4–5, 11–12 and 18-19 for morning and evening milk respectively were analyzed by the Section of Mastitis, National Veterinary Institute, Uppsala, Sweden for bacteriological growth using Mastistrip™ (Department of Mastitis and Diagnostic Products, National Veterinary Institute, Uppsala, Sweden).
Statistical analyses
Analysis of variance was performed on data for 17β-estradiol and lactose in plasma, lactose in urine, milk yield and milk composition using PROC MIXED in the SAS system (SAS Inst. Inc., Cary, NC, USA, version 9.1). Least square means were compared with comparison-wise error rate after significant F-tests. LSD-values were based on calculations with t 0·975. Fixed effect of the day in trial was included in the model (Model 1) used for all parameters except for SCC in milk. For SCC the regression of daily milk yield was included in the model in order to test if there was an effect on SCC beyond the concentration effect caused by variation in milk yield (Model 2). Different autoregressive covariance structures for the within subject variation were tested.
Model 1
Proc mixed; classes cow day; model x = day; repeated/subject = cow type AR(1)
Model 2
Proc mixed; classes cow day; model x = day yield; repeated/subject = cow type AR(1)
Results
17β-estradiol in plasma
Immediately after injections with 17β-estradiol the cows showed a pronounced increase in plasma 17β-estradiol (P < 0·001) (Fig. 1). The highest 17β-estradiol level in plasma was found one day after the injections started. Plasma 17β-estradiol differed between treatment days 5–7, 7–8, 8–10 and 10–12 (P < 0·001) (Fig. 1). The 17β-estradiol level in plasma returned to control level within two days of the last injection.
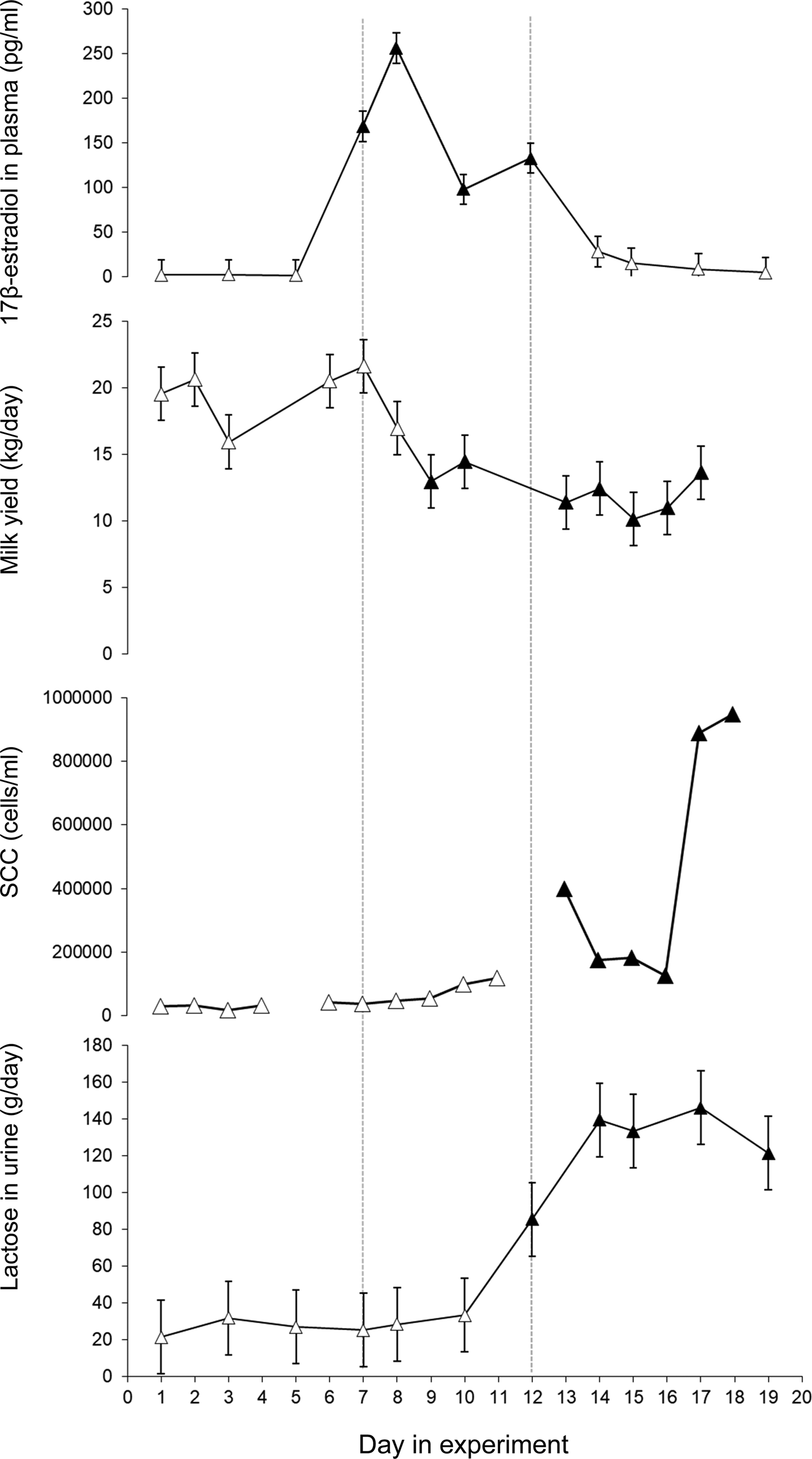
Fig. 1. Least squares mean ± standard error for 17β-estradiol in plasma (pg/ml), milk yield (kg/day), milk somatic cell count (SCC) in morning milk (cells/ml) and lactose in urine (g/day). 17β-estradiol was injected daily during day 7–12. Values that differ significantly (P < 0·001) from the values day 1 and 2 are indicated with filled symbols (▲).
Milk yield and milk composition
The average daily milk yield when the study started was 19·6 ± 3·2 (mean ± sd) (ranging from 15·8 to 24·5 kg). When 17β-estradiol injections started milk yield decreased (P < 0·001) and remained at a lower level until the end of the experiment (Fig. 1). After the injection period some of the cows showed swollen udders which in at least two may have been due to impaired milk ejection. There was no clear response to the exogenous 17β-estradiol treatment in milk lactose or milk fat content. However, milk protein content increased after the treatment period (P < 0·001) and remained elevated until the end of the experiment (Table 1).
Table 1. Coefficient of variation in milk yield and milk protein, lactose and fat content between udder quarters within animals
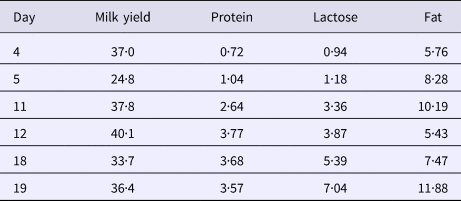
Milk composition varied between udder quarters within animal and the variation between udder quarters in lactose and protein content in milk increased during the experiment whereas the variation in fat content and milk yield was unaffected by the 17β-estradiol treatment (Table 1).
Milk SCC
Milk yield did not explain the changes in milk SCC in the study, therefore Model 1 was used for the ANOVA on milk SCC. When the experiment started SCC was 46 100 (Log 10 = 4·7 ± 0·1) cells/ml in morning milk and 87 700 (Log 10 = 4·9 ± 0·1) cells/ml evening milk. Milk SCC increased in response to the treatment with significantly higher levels in morning milk on days 13–18 (P < 0·05-0·001) and in evening milk on days 12, 13, 15, 17, 18 and 19 (P < 0·05–0·001). The highest mean SCC values were found in the end of the experimental periods, with 945 000 (Log 10 = 5·98 ± 0·2) cells/ml in morning milk and 1 747 000 (Log 10 = 6·2 ± 0·2) in evening milk on the last day of the experiment (Fig. 1 and Supplementary Fig. S1). The bacteriological growth was low and no sign of infection was found in any of the cows during the study.
Lactose in plasma and urine
Lactose was found in plasma and urine in all cows (Fig. 1, Supplementary Fig. S2). The total production of lactose, i.e. the sum of lactose found in milk and in urine, decreased after treatment. The average calculated loss of lactose in urine was 27 ± 19 g/day (mean ± sd) before treatment. The 17β-estradiol treatment gave a pronounced increase in lactose in plasma and urine from day 12 (P < 0·05) and a further increase during days 14 until the study finished on day 19 (P < 0·001) (Fig. 1)) with an average loss of 135 ± 110 g/day (mean ± sd) in urine during days 14–17. The concentration of lactose in urine was linearly related to the plasma lactose concentration (P < 0·001) (Supplementary Fig. S2). The amount of lactose lost in urine before 17β-estradiol injections started accounted for around 4% of the total produced lactose while it increased to 30% of the total lactose in response to the exogenous 17β-estradiol.
Discussion
17β-estradiol injections gave rise to a marked increase in plasma 17β-estradiol. The peak level corresponds to the level of pregnant dry cows about two weeks prior to parturition (Bernier-Dodier et al., Reference Bernier-Dodier, Girard, Talbot and Lacasse2011). The concentration of 17β-estradiol varied during the treatment period even though the cows got the same dose every day (Fig. 1). A regulatory control of 17β-estradiol metabolism that provides buffering against chronic exposure has been suggested (Plowchalk and Teeguarden, Reference Plowchalk and Teeguarden2002). It is possible that, in the present study, 17β-estradiol was metabolized faster after a couple of days of elevated values.
The first response to the increased plasma 17β-estradiol was a drop in milk yield and an increase in milk protein content three days into the treatment period. Low concentrations of lactose were found in both urine and plasma before treatment with exogenous 17β-estradiol, similar to what was found by Schultz et al. (Reference Schultz, Hanisch, Dumke, Springer and Beck1998) in established lactation in cows with good udder health. The observed increase in plasma lactose after 17β-estradiol injections is apparently contradictory to those of Gulay et al. (Reference Gulay, Hayen, Head and Bachman2009) who did not observe any increase in plasma lactose after a 17β-estradiol injection. However, the cows in that study were only followed for 48 h after the injection. In the present study the increase in lactose in plasma was seen after six days of 17β-estradiol injections and four days after the peak in 17β-estradiol in plasma. This delay suggests that 17β-estradiol did not have a direct effect on the tight junctions. It is possible that the first response to the increased plasma 17β-estradiol is reduced mammary lactose synthesis and that the effect on the mammary tight junctions is secondary or that a longer duration of elevated plasma 17β-estradiol is necessary to reduce tight junction integrity. Lactose in plasma and urine remained elevated during the five remaining days of the study, after the treatment with exogenous 17β-estradiol finished, indicating that the elevated 17β-estradiol caused prolonged effects on the mammary epithelium. The varying response to increased plasma 17β-estradiol in lactose and milk protein content between udder quarters indicates that local factors in the mammary tissue are involved in the response to the 17β-estradiol. It has been shown in in vitro studies that 17β-estradiol enhances apoptosis in bovine mammary epithelial cells (Yart et al., Reference Yart, Finot, Lolliver and Dessauge2013), and this may explain the negative effects of 17β-estradiol on milk production. It is also possible that effects on milk ejection seen in the current study are involved in the decreased milk yield. In the current study swollen udders were noted after the treatment period and slower or incomplete milk ejection was suspected. This is supported by the decrease in content of fat and protein in the milk in parallel with the decreased milk yield during the experiment, which would be expected if milk ejection or milk removal was disturbed. An effect caused entirely by lower lactose synthesis would instead give a lower milk yield with higher DM content.
There was an increase in SCC after the 17β-estradiol treatment without sign of infection. It has been suggested that estradiol induces sloughing of mammary epithelial cells which in turn gives rise to high SCC (Moroni et al., Reference Moroni, Pisoni, Savoini, van Lier, Acuña, Damián and Meikle2007). It is also possible that the elevated SCC could play a role in protecting the gland against bacterial invasion during early involution when the gland is highly susceptible to intramammary infection, as has been suggested by Nonnecke and Smith (Reference Nonnecke and Smith1984). It has further been shown that exogenous estrogen enhances innate immune function in the goat mammary gland, which results from an upregulation of innate immune components and a reduction in milk yield (Kuwahara et al., Reference Kuwahara, Yoshimura and Isobe2017). Similar elevations in SCC have been reported also after a single prolonged milking interval (Fox and Schultz, Reference Fox and Schultz1985; Stelwagen and Lacy-Hulbert, Reference Stelwagen and Lacy-Hulbert1996; Lakic et al., Reference Lakic, Wredle, Svennersten-Sjaunja and Östensson2009).
The high correlation between lactose in plasma and urine makes it possible to use only one of these two parameters in further studies. The urine collection proved very easy to perform in this study and collecting urine instead of plasma can be recommended. Also, urine collection gives a possibility to quantify the loss of lactose, in contrast to determinations based on plasma lactose.
In conclusion, the 17β-estradiol injections induced two different responses, the first rapid response was apparently a reduction in lactose synthesis and consequently in milk yield. Secondly, about six days after introduction of the 17β-estradiol injections, the mammary tight junctions became leaky and lactose was thus moved from the milk to plasma and urine via leaky tight junctions. A high correlation between lactose in urine and blood plasma was found.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029919000281
Acknowledgements
The Swedish research council Formas is acknowledged for funding the study (Formas 22·9/2003-0973) and DeLaval International AB in Tumba, Sweden for providing the udder quarter milking machine.






