Introduction
Obsessive–compulsive disorder (OCD) and schizophrenia (SCZ) are two frequent psychiatric diseases that strongly impact the quality of life [Reference Eisen, Mancebo, Pinto, Coles, Pagano and Stout1,Reference Solanki, Singh, Midha and Chugh2]. Among the diversity of symptoms that can be experienced, one key similarity between obsessive thoughts in OCD and delusional ideations in SCZ is that both involve intrusive, unwanted and foreign thoughts [Reference Bottas, Cooke and Richter3,Reference Scotti-Muzzi and Saide4]. Obsessions in OCD are recurrent and persistent thoughts, impulses or images that are experienced as intrusive and inappropriate and that cause marked anxiety or distress. Delusions in SCZ are false beliefs based on incorrect inferences about external reality that are firmly sustained despite what almost everyone else believes and despite what constitutes incontrovertible evidence to the contrary [5]. Although the definitions traditionally suggest the level of insight into the senseless of thoughts to differentiate obsessions in OCD from delusions in SCZ, patients with OCD have been reported to display a wide range of degree of insight about their obsessional thoughts [Reference Kozak and Foa6]. Furthermore, the frequent comorbidity between OCD and SCZ [Reference Buckley, Miller, Lehrer and Castle7,Reference Schirmbeck and Zink8] have led to the definition of new clinical entities, including SCZ with obsessive–compulsive symptoms (up to 30% [Reference Swets, Dekker, van Emmerik-van Oortmerssen, Smid, Smit and de Haan9]), SCZ with OCD (schizo–obsessive disorder, 12–14% [Reference Swets, Dekker, van Emmerik-van Oortmerssen, Smid, Smit and de Haan9,Reference Achim, Maziade, Raymond, Olivier, Mérette and Roy10]), schizotypal personality with OCD (5–50% [Reference Poyurovsky and Koran11]), and OCD with poor insight (13–36%, [Reference Kozak and Foa6,12–20]) [3–5]. These observations suggest a continuum between the obsessive thoughts in OCD and the delusional ideas in SCZ and suggest that these clinical features would share, at least in part, a common cognitive substratum. Among the cognitive deficits that could underpin these two phenomena, it has been hypothesized that patients with OCD [Reference Sher, Frost and Otto21] and patients with SCZ [Reference Brookwell, Bentall and Varese22] would share a failure in their abilities to monitor their own thoughts (source monitoring), leading to confusion between what they actually did and what they only imagined doing.
Source monitoring is a higher-order cognitive process that refers to the ability to remember the source of information. Several subtypes of source monitoring have been identified [Reference Johnson, Hashtroudi and Lindsay23]. A first subtype of source monitoring is reality monitoring, which characterizes the ability to determine whether information was perceived from the environment or imagined (e.g., Did I see my partner close the door or did I only imagine it?). A second subtype of source monitoring is internal source monitoring, which characterizes the ability to distinguish whether an internally generated event was expressed in the outer space or kept in the inner space (e.g., Did I close the door or did I only think about it?). A third subtype is external source monitoring, which refers to the discrimination between different externally derived sources (e.g., Did John tell me that it was going to rain, or did I hear it on the radio?).
Failures in source-monitoring processes have been repeatedly reported in patients with SCZ with particular difficulties in distinguishing internal from external sources of events [24–27]. Both internal source- and reality-monitoring deficits have been found to be associated with positive symptoms, such as delusions [Reference Hoven, Lebreton, Engelmann, Denys, Luigjes and van Holst28,Reference Chiu, Tseng, Chien, Liao, Liu and Yeh29] and hallucinations [Reference Brookwell, Bentall and Varese22,Reference Waters, Woodward, Allen, Aleman and Sommer24,Reference Gawęda, Woodward, Moritz and Kokoszka27].
Only a few studies have investigated source-monitoring abilities in patients with OCD symptoms, compared with the number examining patients with SCZ, and they have provided mixed results [30–34] for positive results and [Reference Sher, Frost and Otto21,35–41] for negative results). This heterogeneous literature has suggested that patients with OCD, especially those with checking symptoms, displayed reduced confidence in internal source-monitoring judgments compared to healthy controls [Reference Lavallé, Brunelin, Bation and Mondino42]. To the best of our knowledge, no studies have directly compared the source-monitoring performances of patients with SCZ with the performances of patients with OCD.
The aim of the present work was therefore to compare patterns of source-monitoring performances between patients with OCD, patients with SCZ, and healthy controls. We hypothesized that patients with OCD will display impaired internal source-monitoring abilities but intact reality-monitoring abilities, whereas patients with SCZ will display alterations in both source-monitoring processes.
Methods
Subjects
We tested a total of 99 participants. Thirty-two patients meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) [5] criteria for OCD, 38 patients meeting DSM-IV-TR criteria for SCZ, and 29 healthy controls (HC), free from any personal or familial neurological or psychiatric condition, were included. At baseline, symptoms were assessed at a screening level, each included patient should not present with other comorbid psychiatric syndrome according to DSM-IV-TR. All participants included presented with intellectual quotient >70. The severity of symptoms was assessed in the OCD group with the Yale-Brown Obsessive–Compulsive Scale (Y-BOCS [Reference Goodman, Price, Rasmussen, Mazure, Delgado and Heninger43]) and in the SCZ group with the Positive and Negative Symptoms Scale (PANSS [Reference Kay, Fiszbein and Opler44]). Patients with OCD and patients with SCZ were on psychotropic medications at the time of testing (Table 1). Duration of illness was 22.3 ± 13.9 for patients with OCD and 13.5 ± 7.8 for patients with SCZ. Patients with SCZ presented with delusional ideation as measured by the PANSS item #P1 (median = 4.0, mean = 4.0, standard deviation = 1.6) and hallucinations as measured by PANSS item #P3 (median = 6.0, mean = 5.5, standard deviation = 1.4). Patients with OCD presented with obsessions (median = 13.0, mean = 12.5, standard deviation = 3.4) as measured with YBOCS items #1–5 and compulsions (median = 13.0, mean = 12.2, standard deviation = 3.6) as measured by the YBOCS items #6–10. Within the five dimensions of OCD symptoms, 1 patient fitted with the symmetry-ordering dimension, 16 patients fitted with the contamination-washing dimension, 6 patients fitted with the sexual-religious dimension, 9 patients fitted with the aggressive obsession-checking dimension, and 0 patients fitted with the hoarding dimension. All participants were native French speakers and provided written informed consent after a detailed description of the study. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Table 1. Sociodemographic and clinical characteristics of the included population.

The results are given as the mean ± standard deviation.
Abbreviations: HC, healthy controls; OCD, obsessive–compulsive disorder; SCZ, schizophrenia.
The sociodemographic and clinical characteristics of the population are given in Table 1.
Source-monitoring tasks
The testing procedure took approximately 15 min to complete. The participants were individually tested with two source-monitoring tasks presented in a randomized order: one internal source-monitoring task and one reality-monitoring task. Each source-monitoring task was divided into two phases, an encoding phase and a memory retrieval phase, which were preceded by a short practice trial. As displayed in Figure 1, during the encoding phase, 16 words were sequentially presented in a randomized order. Each word was preceded by an instruction. During the memory retrieval phase, 24 words were presented, including the 16 words that were presented during the encoding phase and 8 new words. Our stimulus materials were similar to those described by Brunelin et al. [Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery45].
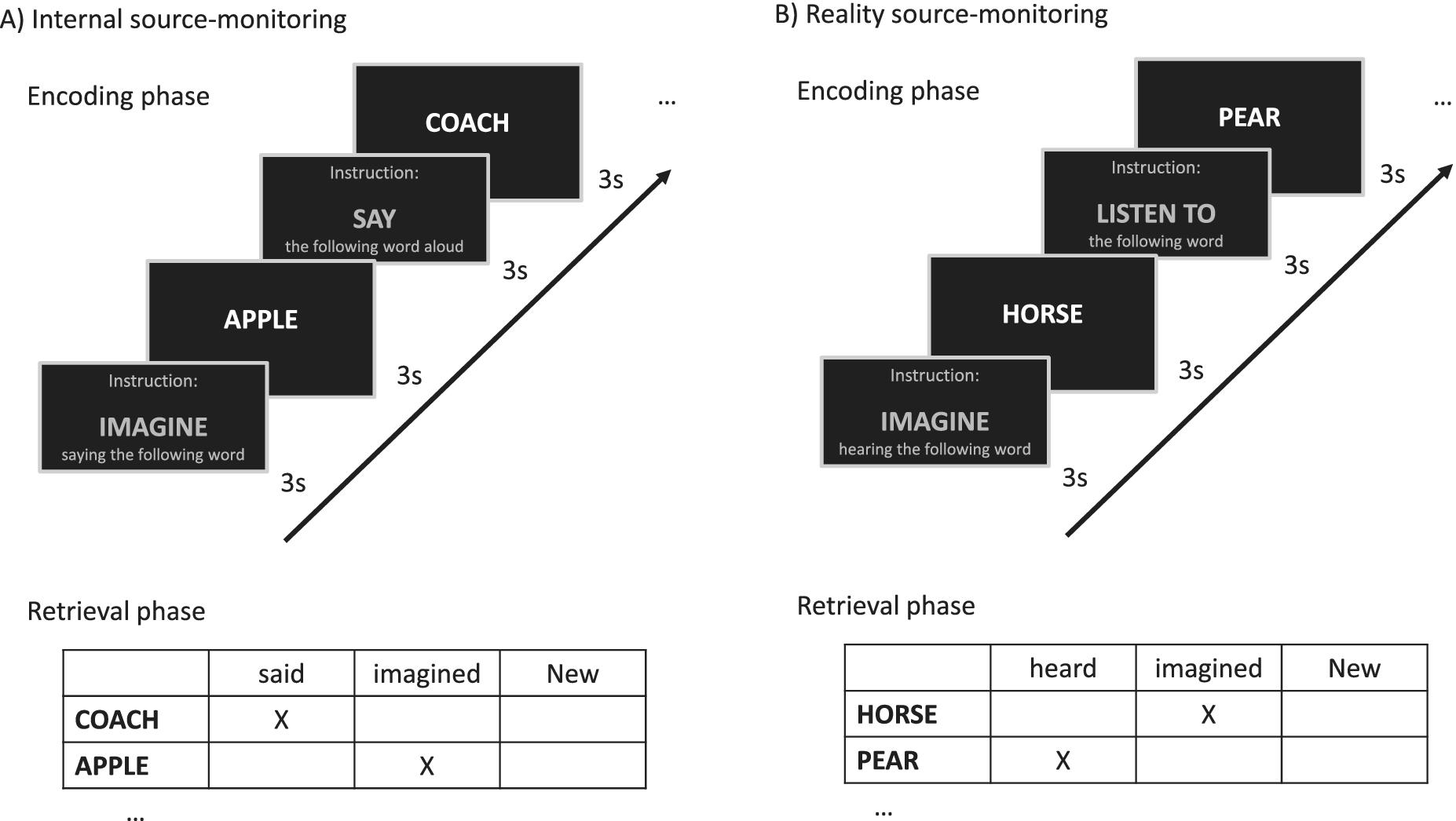
Figure 1. Experimental procedures for reality-monitoring (right panel) and internal source-monitoring (left panel) assessments.
Internal source monitoring
The verbal list included eight words with the “imagine saying” encoding condition and eight other words with the “say aloud” encoding condition. Then, internal source-monitoring abilities were assessed by asking subjects to identify whether each word was said aloud, imagined or new (Figure 1A).
Reality monitoring
The verbal list included eight words with the “imagine hearing” encoding condition and eight other words with the “listen to” encoding condition. Then, reality-monitoring abilities were assessed by asking subjects to identify whether each word was heard, imagined or new (Figure 1B).
Analyses
Statistical analyses were carried out using SPSS software (version 26.0). Statistical significance was set at p < 0.05, and eta-squared (η 2) was also reported.
The differences in age and educational level among groups were examined using one-way ANOVA tests. Gender differences were examined using Pearson’s chi-squared test.
Primary outcomes were correct responses in the source-monitoring tasks. Internal source-monitoring correct responses were the number of said words that were correctly identified as said (SAY), the number of imagined words that were correctly identified as imagined saying (IMA), and the number of unpresented words that were correctly identified as new (NEW). Reality-monitoring correct responses were the number of heard words correctly identified as heard (HEAR), the number of imagined words correctly identified as imagined hearing (IMA), and the number of unpresented words correctly identified as new (NEW).
Correct responses were analyzed using two-way repeated-measure ANOVA models with group (OCD, SCZ, and HC) as a between-subject factor and response type (HEAR, IMA, and NEW for reality-monitoring assessment; SAY, IMA, and NEW for internal source-monitoring assessment) as a within-subject factor. When significant, ANOVAs were followed by Fisher LSD post hoc tests. ANCOVAs were performed when sociodemographic parameters significantly differed between groups.
Results
Demographic characteristics
No significant difference between groups was found for age (p = 0.46) or gender (p = 0.10), but a significant difference was observed for educational level (p = 0.03). Post hoc tests showed a significant difference between subjects with SCZ and HC (p = 0.02) but no significant difference between subjects with OCD and HC (p = 0.15) or between subjects with SCZ and subjects with OCD (p = 0.73).
Internal source monitoring
For internal source-monitoring correct responses, two-way repeated measures ANOVA revealed a significant effect of group (F (2,96) = 8.39, p < 0.001, η 2 = 0.149) and response type (F (2,96) = 104.34, p < 0.001, η 2 = 0.52) and a significant interaction between these two factors (F (4,96) = 5.04, p = 0.001, η 2 = 0.09). Repeated measures ANCOVA revealed a significant effect of educational level on internal source-monitoring correct responses (F (2,96) = 4.123, p = 0.019, η 2 = 0.081). The interaction between group and response type remained significant after including educational level in the model (F (4,96) = 4.357, p = 0.002, η 2 = 0.084).
Post hoc tests revealed no significant differences among the groups for SAY and NEW correct responses (all p > 0.05). The patients with SCZ made significantly fewer IMA correct responses than both the patients with OCD (p = 0.04, η 2 = 0.05) and HC (p < 0.001, η 2 = 0.28), while the patients with OCD presented an intermediate level of deficit (OCD–HC comparison: p = 0.02, η 2 = 0.13) (Figure 2).

Figure 2. Comparison of internal source-monitoring types of correct responses between HC (dark gray bars), patients with OCD (light gray bars), and patients with SCZ (medium gray bars). SAY responses are measured as the number of correctly identified said words, IMA responses are measured as the number of correctly identified imagined words and NEW responses are measured as the number of correctly identified new words. The results are displayed as the mean ± standard deviation. ns, not significant. ***p < 0.001; **p < 0.01; *p < 0.05.
Reality monitoring
For reality-monitoring correct responses, two-way repeated measures ANOVA revealed a significant effect of group (F (2,96) = 9.71, p < 0.001, η 2 = 0.17) and response type (F (2,96) = 77.47, p < 0.001, η 2 = 0.45) with no significant interaction between these two factors (F (4,96) = 2.34, p = 0.056, η 2 = 0.05). Repeated measures ANCOVA revealed no significant effect of educational level (p = 0.17) on reality-monitoring correct responses.
Post hoc comparisons among the groups revealed no significant differences in the number of NEW correct responses (all p > 0.05). Patients with SCZ made significantly fewer IMA correct responses than both patients with OCD (p = 0.01, η 2 = 0.59) and patients with HC (p = 0.001, η 2 = 0.91), with a significant difference between patients with OCD and HC (p = 0.25, η 2 = 0.03). Patients with SCZ made fewer HEAR correct responses than HC (p = 0.037, η 2 = 0.65) with no significant difference with patients with OCD (p = 0.07, η 2 = 0.44) and between patients with OCD and HC (p = 0.79, η 2 = 0.06) (Figure 3).
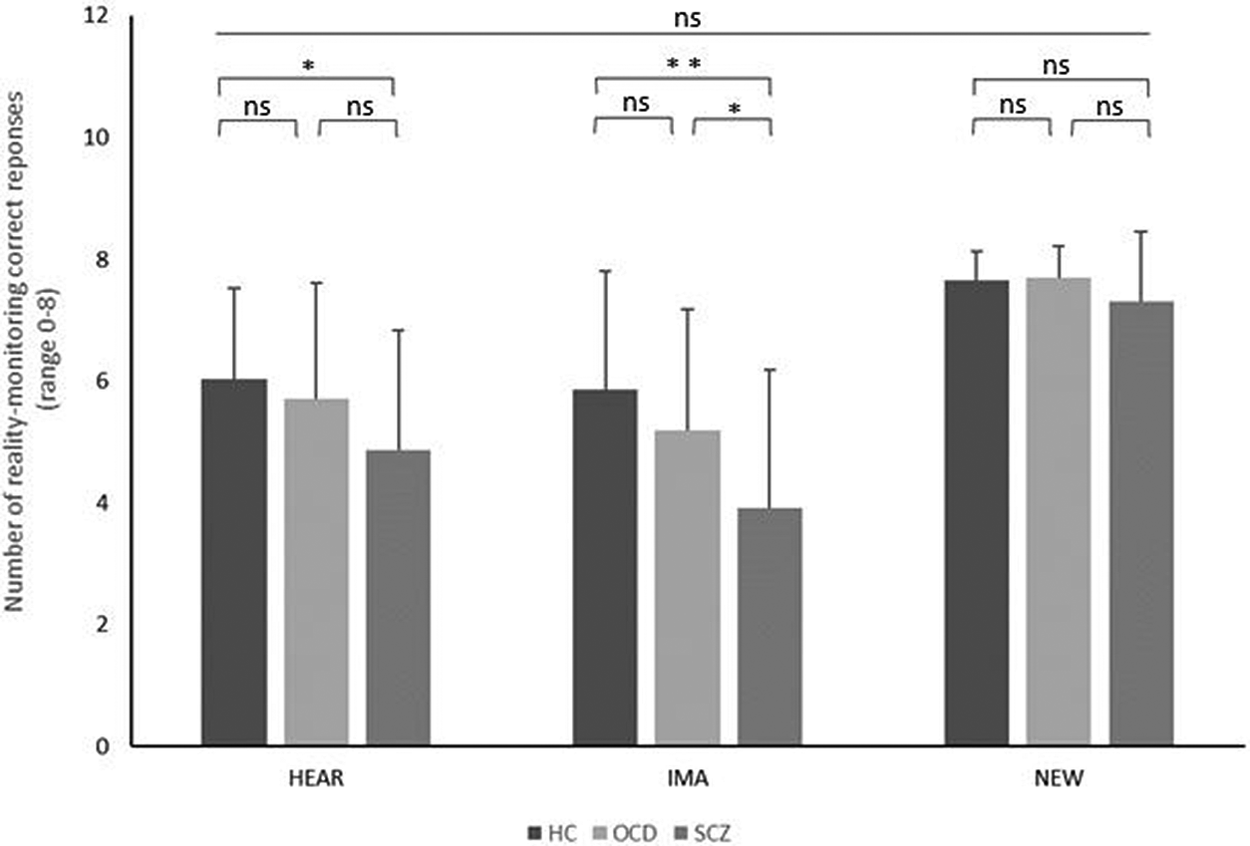
Figure 3. Comparison of reality-monitoring correct responses between HC (dark gray bars), patients with OCD (light gray bars), and patients with SCZ (medium gray bars). HEAR responses are measured as the number of correctly identified heard words, IMA responses are measured as the number of correctly identified imagined words, and NEW responses are measured as the number of correctly identified new words. The results are displayed as the mean ± standard deviation. ns, not significant. ***p < 0.001; **p < 0.01; *p < 0.05.
Discussion
The aim of the study was to compare source-monitoring abilities between patients with OCD, patients with SCZ, and HC. We reported a dissociation between psychiatric condition and source-monitoring impairments. Namely, both the patients with SCZ and OCD showed impaired internal source-monitoring performances compared to the HC. Performance of patients with OCD was intermediate between that of the HC and patients with SCZ. In contrast, only the patients with SCZ showed impaired reality-monitoring performances compared to the HC.
In the internal source-monitoring task, the patients with OCD and patients with SCZ were less likely to recognize the words they had to imagine saying than the HC. However, they made a similar number of correct responses regarding said words and new words as the HC. These results suggested that OCD and SCZ share a common deficit in the recognition process of what they actually did and imagined doing. This result is in line with previous studies in patients with SCZ that observed internal source-monitoring deficits in patients [Reference Keefe, Arnold, Bayen, McEvoy and Wilson46,Reference Humpston, Linden and Evans47] suggesting that a failure in monitoring their own thoughts may contribute to SCZ positive symptoms such as delusions and hallucinations. As a comorbid diagnosis of SCZ was ruled out in our OCD sample, one can hypothesize that the observed internal source-monitoring deficits may similarly contribute to both OCD and SCZ symptoms. Traditionally, the obsessional thoughts in OCD have been distinguished from the delusions and hallucinations in SCZ because of their lack of perceptual qualities and the high level of patients’ insight. However, numerous studies have recently reconsidered this dichotomy by showing that patients with OCD have a wide range of insight levels [Reference Kozak and Foa6,48–51] and frequently described their obsessional thoughts as perceptual irritations [52–55]. Moreover, patients with SCZ with diagnosed hearing hallucinations often describe them as less loud than real voices or without perceptual qualities but merely as strange thoughts [Reference Moritz and Larøi56]. Even if it remains speculative, these results suggest that an internal source-monitoring deficit may contribute, at least in part, to the phenomenological overlap between delusions/hallucinations and obsessional intrusive thoughts. Within this hypothesis, the deficit in internal source monitoring may lead to uncertainty regarding the source of self-generated events, and this uncertainty may contribute to the obsessive thoughts in OCD as well as to the positive symptoms in SCZ. The shared alterations in internal source monitoring in OCD and SCZ is consistent with the clinical overlap between these two, including frequent comorbidities and a wide range of schizo–obsessive spectrum disorders [Reference Scotti-Muzzi and Saide4]. To clarify the link between internal-monitoring scores and specific clinical features, further studies should consider evaluating source-monitoring as a transdiagnostic factor by assessing SCZ delusional symptoms in the OCD sample, OCD obsessional thoughts in the SCZ sample and analyzing correlations between source-monitoring performances and these clinical scores.
Despite shared abnormalities, the patients with OCD were significantly less impaired in internal source monitoring than the patients with SCZ. This gradation suggests that thought-recognition deficits are a cognitive dimension common to yet differentially impaired in patients with SCZ and OCD. This result is in line with earlier findings that both patients with OCD and SCZ were impaired in several neuropsychological domains (memory, attention, visual spatial, and executive functioning) with more pronounced deficits in patients with SCZ [Reference Martin, Huber, Rief and Exner57,Reference Whitney, Fastenau, Evans and Lysaker58]. In the future, transdiagnostic evaluations of both OCD and SCZ symptoms should help clarifying the relationship between the gradation of internal source-monitoring scores and clinical dimensions. Furthermore, it is worth noting that the deficit in recognizing imagined items is associated with significant forgetting bias (i.e., erroneous classification of previously presented imagined items as old) in both patients with OCD and patients with SCZ (supplementary material S1). Patients with SCZ additionally displayed significant externalization bias (i.e., misattributing a self-generated item to an external source). Further studies with larger samples are warranted to validate these findings and explore their implications.
Regarding reality monitoring, the patients with OCD showed similar results to the HC and displayed significantly better results than the patients with SCZ. In other words, since both groups of patients were impaired in internal source monitoring, the only OCD group had preserved recognition of self-generated imagined words when contrasted with externally perceived words. Conversely, the patients with SCZ were additionally impaired to distinguish internally generated sources from externally perceived ones. This result is consistent with numerous studies showing that patients with SCZ display a deficit in reality monitoring. Specifically, SCZ studies have suggested internal source-monitoring deficits as a consistent trait of the disorder and associated the presence of misattributions of agency in hallucinations with more marked reality source monitoring [Reference Brunelin, Combris, Poulet, Kallel, D’Amato and Dalery45]. However, it is interesting to note that patients with SCZ did not showed any externalization bias but forgetting bias, which denote strongly with previous findings [Reference Brookwell, Bentall and Varese22]. In contrast, the finding of intact reality-monitoring performances in the patients with OCD seems in line with clinical observations that have reported that obsessive thoughts are generally ego-dystonic (i.e., patients recognize their intrusive and inappropriate quality [5]). The unshared reality-monitoring impairment between patients with OCD and SCZ thus provides support for the assumption that reality-monitoring impairments are specific to patients with SCZ and the misattribution of inner thoughts.
Our results should be interpreted with caution because of several limitations. First, this study suffers from a lack of control of insight levels. It is admitted that patients with SCZ hold their delusional beliefs as probably true and integrated into their belief system. In contrast, it is commonly described that patients with OCD judge their obsessive thoughts as intrusive, disturbing or senseless. However, poor insight affects between 13 and 36% of patients [Reference Kozak and Foa6,12–20] and has been negatively correlated with the perceptual qualities that patients attribute to obsessive thoughts [Reference Moritz, Claussen, Hauschildt and Kellner52]. Furthermore, compared to patients with OCD who have normal levels of insight, patients with OCD who have poor insight present a higher rate of SCZ spectrum disorder in their third-degree relatives [Reference Catapano, Sperandeo, Perris, Lanzaro and Maj49], a higher comorbidity with schizotypal personality disorder [Reference Matsunaga, Kiriike, Matsui, Oya, Iwasaki and Koshimune14], and more severe OCD symptoms [Reference Catapano, Perris, Fabrazzo, Cioffi, Giacco and De Santis17]. Accordingly, patterns of source-monitoring deficits in patients with SCZ may be more similar to patients suffering from OCD with poor insight than with normal insight. For these reasons, insight levels might be an important confound and should be integrated as a covariable in future source-monitoring studies involving patients with SCZ and OCD. The relationship between internal-source monitoring performances and insight level in patients with OCD should also be specifically investigated in further studies. Second, the small sample of this study did not allow for performing subgroup comparisons of source-monitoring performances between the different clinical profiles of patients with OCD (i.e., symmetry-ordering, contamination-washing, sexual-religious, aggressive obsession-checking, and hoarding). Third, our study revealed different educational levels between the SCZ and HC groups. Even if educational level has been repeatedly demonstrated to interfere with memory functions [59–61], no changes were observed in source-monitoring differences when education was entered in ANCOVAs. Hence, it would be relevant to explore the relationship between level of education and memory, when memory process involves discrimination between two sources. Fourth, one can hypothesize that source-monitoring effects are a consequence of a general memory deficit or a lack of confidence in memory in the two populations of patients compared to the HC. While this is unlikely that general memory/confidence deficits are responsible for specific impairment in recognizing imagined items, these factors would be tested and analyzed in further studies. Fifth, the different types of medication regimens between the patients with OCD and patients with SCZ may be considered a confounding factor. In a small sample of patients with SCZ, antipsychotic intake has been associated with reduced reality-monitoring impairment [Reference Keefe, Poe, McEvoy and Vaughan62]. As far a swe know, no similar studies have explored the potential impact of pharmacological agents on source-monitoring in patients with OCD. However, in a study comparing source-monitoring abilities between patients with SCZ, patients with major depressive disorder (MDD) and HC, no significant source-monitoring deficit was showed in patients with MDD treated by Selective Serotonin Reuptake Inhibitors (SSRI) [Reference Brunelin, Poulet, Marsella, Bediou, Kallel and Cochet63]. This finding suggests that SSRI do not have an impact on source-monitoring performance in our OCD sample (see Table 1). Since several studies have also reported that impairments of other cognitive functions associated with OCD and SCZ are independent of medication type and dosage [64–66], investigating if and how medication status could influence source-monitoring in these two population of patients seems warranted in the future. Further studies involving first-episode and drug-naïve patients may also provide more reliable evidence regarding the influence of medication on source-monitoring performances. Sixth, future research should include some evaluation of additional neurocognitive parameters, such as working memory, executive functions, and metacognitive parameters that might influence source-monitoring scores. Finally, it should be noted that there is a relatively low number of events in both source-monitoring paradigms, which prevent us from running correlations between source-monitoring and clinical scores without maximizing the risk of type II error. Future studies are warranted to validate the present findings with a larger number of stimuli.
Our results are in line with previous studies that showed that patients with SCZ displayed a dual deficit in internal source and reality monitoring. Using a categorical approach, our study points for the first-time internal source-monitoring as a transdiagnostic factor between patients with OCD and SCZ. In contrast, reality-monitoring performance distinguishes patients with OCD from patients with SCZ. A dimensional approach would be warranted in the future to explore the relationship between source-monitoring performances and specific clinical features that overlap between OCD and SCZ. Furthermore, the pattern of source-monitoring impairments reported here implies disturbances in partially overlapping brain systems in OCD and SCZ that future researches should identify.
Acknowledgments
The authors thank the Vinatier Hospital Center for its financial support.
Financial Support
This study was supported by a Scientific Research Council grant from the Vinatier Hospital Center (Bron, France). The funding source had no further role in the data collection, analysis and interpretation; in the study design; in the writing process; and in the decision to submit the manuscript for publication.
Conflict of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Data Availability Statement
There are no linked research data sets. Data will be made available on request.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1192/j.eurpsy.2020.48.







Comments
No Comments have been published for this article.