The development of internal and external genitalia starts from the same baseline embryological point. From the ninth week of gestation, it diverges to differentiate into either male or female, depending on chromosomes, genes and hormones. The development of internal female genitalia is closely linked to that of the urinary tract; hence relevant details of urinary tract embryology will be outlined in this chapter.
1.1 Control of Sex Differentiation and Genetics
In species with heteromorphic sex chromosomes, such as human beings, sex differences arise from the genetic differences found in the sex chromosomes. The numerous sex-specific and sex-biased factors that interact in the network of genes and molecules and result in sexual differentiation are called sexome [Reference Arnold and Lusis1]. Female-biasing factors include two X chromosomes, ovarian hormones; male-biasing factors include a single X chromosome, the Y chromosome, and testicular hormones. The primary sex-determining factors are encoded by the sex chromosomes and are the only factors that differ in the male and female zygote. The secondary factors involve genes that are coded in the autosomal chromosomes [Reference Arnold and Lusis1,Reference Arnold, Chen and Itoh2].
The key role in sex differentiation in male development is played by SRY (sex-determining region on Y chromosome), a transcription factor derived from the short arm of the Y chromosome (Yp11). SRY initiates a cascade of downstream genes that determine the male development. It acts directly on the gonadal ridge and indirectly on the mesonephric duct for the development of the testes. It also causes the activation of genes that inhibit ovarian differentiation, and it upregulates steroidogenesis factor 1 (SF1), which through the SOX9 gene causes the differentiation of Sertoli and Leydig cells [Reference Larsen3,Reference Catherine and Rien4].
Absence of SRY in conjunction with positive mediation by specific genes on X chromosome causes the zygote to develop into a female. The X-linked and autosomal genes initiate ovarian development and block testicular differentiation. The two main genes involved in female sexual differentiation are DAX1 and WNT4. DAX1 is a member of the nuclear hormone receptor family located on the short arm of the X chromosome and acts by downregulating SF1 activity. WNT4 is a growth factor early expressed in the genital ridge that is maintained only in females and contributes to ovarian differentiation [Reference Arnold, Chen and Itoh2,Reference Larsen3,Reference Blaschko, Cunha and Baskin5].
In addition to genes, sexual differentiation is affected by the hormonal milieu of the developing baby and end receptor sensitivity to hormones. Abnormal hormonal production by the placenta or adrenal cortex, or extraneous hormonal influence, or receptor insensitivity to hormones can affect sexual development, which may be contrary to that which would be expected from genetic sex.
1.2 Stages of Sex Differentiation
1.2.1 Early Development of the Zygote
Organogenesis occurs in the first 10 weeks of gestation and the remaining 28 weeks are spent in maturation, growth and development of function.
After fertilisation, the developing zygote divides and forms the blastocyst (Figure 1.1a). Later, two cavities – the amniotic cavity and the yolk sac – develop. The embryo arises from two layers of cells interposed between these two cavities, ectoderm and endoderm (Figure 1.1b). At approximately 15 days, an ingrowth of cells from the primitive streak forms a third layer between them, the mesoderm (Figure 1.1c). At the head and tail ends of the embryo, the mesoderm is deficient, resulting in the development of the buccopharyngeal and the cloacal membrane, respectively. The mesoderm is divided into three parts: lateral plate mesoderm, intermediate mesoderm and paraxial mesoderm (Figures 1.1d and 1.2a). Gonads, kidneys and genital ducts develop from the urogenital ridge on the intermediate mesoderm (Figure 1.2b). Definitive kidneys develop from the nephrogenic cord (Figure 1.2c), which is divided craniocaudally into pronephros (primitive kidney – disappears)/mesonephros (intermediate kidney – disappears)/metanephros (definitive kidney). Two symmetrical pairs of genital ducts – mesonephric (Wolffian) and paramesonephric (Müllerian) ducts – develop lateral to the nephric blastema (or nephrogenic cord) (Figure 1.2c) and give rise to internal male and female genitalia. Gonads develop anteromedial to the mesonephros, from the genital ridges (Figure 1.2c).

Figure 1.1 Early zygote development. (a) From fertilisation to implantation, first 5–6 days. (b) Development of two cavities – the amniotic cavity and the yolk sac – and bilaminar embryonic disc: endoderm and ectoderm. (c) Formation of third layer (mesoderm) from primitive streak. (d) Differentiation of mesoderm into paraxial, intermediate and lateral plate mesoderm. (e) Cephalocaudal folding of the embryo and development of early bladder (allantois), defining cloaca as part of hindgut distal to allantois. (f) Division of cloaca into urogenital sinus and anorectal canal by urorectal septum.
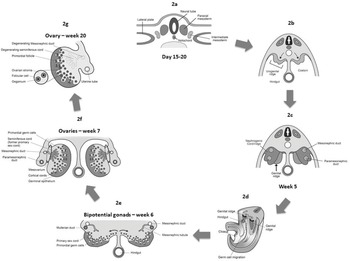
Figure 1.2 Gonadal development. (a, b) Cross section of the embryo showing intermediate mesoderm, which gives rise to the urogenital ridge. (c) Urogenital ridge differentiating into nephrogenic cord laterally and genital ridge medially. (d) Longitudinal section of the embryo showing migration of primordial germ cells into the genital ridge, from the yolk sac along the wall of the hindgut and the dorsal mesentery. (e) Cross section showing germ cell penetration into the genital ridges. (f) Early phase of ovarian development. (g) Advanced phase of ovarian development with degeneration of mesonephric duct.
Between the third and fourth weeks of gestation, head and tail ends of the embryo fold cephalocaudally. The endoderm of the yolk sac is included within the two folds and forms the gut. The allantois gains continuity with the developing gut and delimits the cloaca as the portion of hindgut distal to their confluence (Figure 1.1e). Between the fourth and sixth weeks of gestation, the cloaca is subdivided into the primitive urogenital sinus anteriorly and the anorectal canal posteriorly by the descent of the urorectal septum, from the point of confluence of allantois and hindgut, towards the cloacal membrane/perineum and laterally by the folds of Rathke (Figure 1.1f) [Reference Larsen3,Reference Gearhart, Rink and Mouriquand6,Reference Thomas, Duffy and Rickwood7].
1.2.2 Development of Gonads
Gonads appear as a pair of longitudinal ridges (genital or gonadal ridges) sited on the anteromedial aspect of the mesonephros, the intermediate kidney (Figure 1.2c). Derived from intermediate mesoderm and overlying epithelium, these ridges initially do not contain germ cells.
During the third week of gestation, primordial germ cells appear on the wall of the yolk sac close to the allantois. Subsequently, they migrate along the dorsal mesentery of the hindgut (Figures 1.2d and 1.2e) to the primitive gonads (fifth week). During the migration they proliferate through mitosis and in the sixth week they invade the genital ridges (Figures 1.2d and 1.2e). Throughout this period, the epithelial cells of the genital ridge proliferate and penetrate the underlying mesenchyme, forming the primitive sex cords. At this stage, the gonad is undifferentiated (Figure 1.2e). The primitive sex cords and the primordial germ cells are found in both the cortical and the medullary zone and it is not possible to distinguish between male and female gonads. The initial formation of the bipotential gonad requires two transcription factors: Wilms’ tumour 1 (WT1) and SF1. SRY is pivotal in further sexual determination and interplays mainly with two genes: SOX9 and DAX1, determining the differentiation into testis or ovary.
Due to their inductive influence on the development of gonad into ovary or testis, if the germ cells fail to reach the ridges, the gonads do not differentiate. From the sixth week, differentiation of gonads into testis or ovary occurs. In XX embryos the ovary will originate from the cortex and medulla will decline. In the XY embryo, medulla will develop into testis and cortex regresses [Reference Larsen3,Reference Gearhart, Rink and Mouriquand6–Reference Makiyan8].
1.2.2.1 Ovary
The ovary develops later than the testis and until the tenth to eleventh week, it does not have distinguishable histological features.
Once primordial germ cells have arrived in the gonad of a genetic female, they differentiate into oogonia and undergo several mitotic divisions (Figures 1.2e and 1.2f). In the meantime, in the presence of XX chromosomes and absence of SRY gene, the primitive sex cords degenerate. Instead, the epithelium of the gonad continues to proliferate producing secondary sex cords called cortical cords, that extend from the surface epithelium. As these cords increase in size, the primordial germ cells are incorporated into them. In females, the secondary sex cords retain their connection to surface epithelium and, therefore, the primordial germ cells are mainly found in the cortex (Figures 1.2e and 1.2f). Only a few of the sex cords reach the medulla, but those that go into depths and lose contact with the coelomic epithelium tend to undergo atrophy.
Oogonia proliferate by mitosis and then enter the first prophase of meiotic division to form primary oocytes. By the end of the third month of gestation, the oogonia are surrounded by a single layer of flattened epithelial cells which constitute the supporting cell lineage (granulosa or follicular cells) and are arranged in clusters (Figure 1.2g). By the fifth month of prenatal development, the total number of germ cells in the ovary reaches its maximum at about 7 million. However, many germ cells are lost during development. By the seventh month, all the surviving germ cells have entered meiosis and further germ cell development is arrested until puberty. A primary oocyte, together with its surrounding flat epithelial cells, is known as primordial follicle. The number of primordial follicles at birth amount to between 300,000 and 2 million, decreasing to 40,000 at puberty. Only about 300 primary oocytes develop further between puberty and menopause into fertilisable oocytes [Reference Larsen3,Reference Thomas, Duffy and Rickwood7,Reference Makiyan8].
1.2.2.2 Developmental Anomalies
Turner syndrome is a chromosomal condition that affects ovarian development in females. It can be caused either by monosomy X or by X chromosome mosaicism and results in early loss of ovarian function (ovarian hypofunction or premature ovarian insufficiency) due to premature death of oocytes and degeneration of ovarian tissue. Many affected girls do not undergo puberty unless they receive hormone therapy, and most have significantly reduced fertility [Reference Thomas, Duffy and Rickwood7,Reference Makiyan8].
1.2.2.3 Testis
The testis develops earlier than the ovary. In the presence of XY chromosome complex, the SRY encodes for the testis-determining factor (TDF) and regulates the proliferation of the primitive sex cords and their penetration into the medulla to form the testis. A part of these cords forms the future rete testis and the other part containing germ cells and Sertoli cells becomes seminiferous tubules. The Leydig cells, located between the cords, start producing testosterone from the eighth week, driving the differentiation of internal and external genitalia [Reference Gearhart, Rink and Mouriquand6,Reference Thomas, Duffy and Rickwood7].
1.2.3 Differentiation of Internal Genital Organs and Ducts
1.2.3.1 Molecular Regulation of Genital Duct Development
The male and female genital tract is undifferentiated until the ninth week of development. The mesonephric and paramesonephric ducts together with the urogenital sinus give origin to the internal genitalia.
Female sexual differentiation, hitherto thought to be a default mechanism that occurs in the absence of a Y chromosome, is an active process mediated by specific genes on X chromosome. DAX1 downregulates the SF1 activity, preventing the differentiation of Sertoli and Leydig cells in gonads. The growth factor WNT4 contributes to ovarian differentiation. In the absence of MIS (Müllerian inhibiting substance, also called anti-Müllerian hormone/AMH), the paramesonephric ducts are stimulated by oestrogens to form the fallopian tubes, uterus, cervix and upper vagina. Oestrogens also act on the external genitalia.
In male embryos, SRY induces testicular development and differentiation of Sertoli and Leydig cells. They produce MIS and testosterone, respectively. AMH causes regression of the paramesonephric ducts, while testosterone and its derivative dihydrotestosterone (DHT) mediates development of the mesonephric ducts into epididymis, rete testis, vas deferens, ejaculatory ducts and seminal vesicles. It also induces differentiation of the male external genitalia [Reference Larsen3,Reference Blaschko, Cunha and Baskin5,Reference Thomas, Duffy and Rickwood7].
1.2.3.2 Genital Duct Differentiation
The paramesonephric duct emerges as a longitudinal invagination of the epithelium on the anterolateral surface of the urogenital ridge (Figure 1.3a). Cranially, the duct opens into the abdominal cavity with a funnel-shaped structure (abdominal ostium of the fallopian tubes) and it runs lateral to the mesonephric duct. Caudally, it crosses ventrally and towards the midline, coming in close contact with the paramesonephric duct from the opposite side. The two ducts meet and fuse in a Y shape, forming the utero-vaginal duct (Figure 1.3b). Initially the two ducts are separated by a septum, but later on this vanishes and forms the uterine canal (ninth week). The point of contact of the Müllerian ducts with the urogenital sinus is called Müllerian tubercle (Figure 1.3b). The mesonephric ducts open into the urogenital sinus on either side of the Müllerian tubercle and later regress in the female (Figure 1.3c).
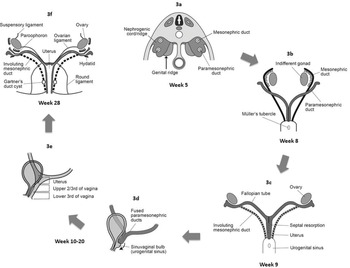
Figure 1.3 Paramesonephric duct development. (a) Paramesonephric duct arising as invagination of peritoneum over the nephrogenic ridge. (b) Distal part of paramesonephric ducts join in the midline. (c) The unfused part of paramesonephric ducts forms the fallopian tubes; the distal fused part gives rise to the uterus and the upper two-thirds of the vagina. (d, e) Development of the vagina from distal fused paramesonephric ducts and urogenital sinus. (f) Regression of mesonephric ducts with embryonic remnants proximally and distally. Peritoneal fold forming the ovarian ligament and round ligament of the uterus.
After the ducts fuse distally in the midline, a broad transverse peritoneal fold is established. This fold, which extends from the lateral sides of the fused paramesonephric ducts towards the wall of the pelvis, is the broad ligament of the uterus. The fallopian tube lies in its upper border and the ovary lies on its posterior surface. The uterus and broad ligaments divide the pelvic cavity into an anterior and a posterior pouch, respectively the uterovesical and the uterorectal pouch. Remnants of the mesonephric ducts are common in the broad ligament (Figure 1.3f).
The fused paramesonephric ducts give rise to the corpus and cervix of the uterus (Figures 1.3d and 1.3e). They are surrounded by a layer of mesenchyme that forms the muscular wall of the uterus, the myometrium, and its peritoneal covering, the perimetrium [Reference Sajjad9,Reference Robboy, Kurita, Baskin and Cunha10].
The vagina has a dual origin, the upper two-thirds derive from the paramesonephric ducts, whereas the lower third derives from the urogenital sinus (Figure 1.3e). Shortly after the paramesonephric ducts reach the urogenital sinus, two solid evaginations grow out from its posterior wall creating the sinovaginal bulbs (Figure 1.3d). These proliferate and form a solid vaginal plate, which elongates and canalises by the twentieth week, giving rise to the lower third of the vagina. Between the third and fifth months, proliferation continues at the cranial end of the plate, increasing the distance between the uterus and the definitive vestibule (Figure 1.3e). The lumens of the vagina and of the definitive urogenital sinus are separated by a thin tissue plate which will partially degenerate after the fifth month. Its remnant, the hymen, consists of the epithelial lining of the sinus and a thin layer of vaginal cells. During perinatal life it develops a small opening. If this fails, an imperforate hymen or variations thereof, such as septate or microperforate, can develop. The urogenital sinus caudal to the vaginal opening becomes the vestibule [Reference Larsen3,Reference Thomas, Duffy and Rickwood7,Reference Cunha, Robboy, Kurita, Isaacson, Shen and Cao11,Reference Walsh, Retik, Vaughan and Wein12].
1.2.3.3 Uterine and Vaginal Developmental Anomalies
Incomplete fusion of the Müllerian ducts gives rise to a variety of uterine and vaginal anomalies depending on the level of the anatomical defect, whether limited or throughout the entire line of fusion.
Uterus didelphys develops from complete failure of fusion of the paramesonephric ducts, partial failure results in bicornuate uterus or arcuate uterus. The lack of fusion of the two sinovaginal bulbs causes development of a double vagina. In contrast, lower vaginal atresia occurs if the bulbs do not develop at all. In this case a small vaginal pouch derived from the caudal part of the paramesonephric ducts surrounds the opening of the cervix. Defect in canalisation causes transverse vaginal septa and atretic segments of vagina.
Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome is a rare disorder characterised by incomplete development of the Müllerian duct. It results in failure of uterus and vagina maturation and these may be atretic or absent. Women with this condition have normal ovarian function and external genitalia. They develop normal secondary sexual characteristics during puberty, but have primary amenorrhea.
Vestigial remnants of the mesonephric ducts may remain at proximal and distal ends and are usually found in broad ligament and adjacent to vagina. They are the epoophoron and paroophoron and the duct of Gartner in the wall of the vagina (Figure 1.3f). These can undergo cystic malformation later in life resulting in paraovarian cysts and Gartner cysts [Reference Thomas, Duffy and Rickwood7,Reference Cunha, Robboy, Kurita, Isaacson, Shen and Cao11,Reference Walsh, Retik, Vaughan and Wein12].
1.2.3.4 Descent of the Ovaries
Gonads, together with the kidneys, develop retroperitoneally. During fetal growth, both female and male gonads undergo anatomical descent, the ovaries moving into the pelvis and the testes into the scrotal sacs. Descent of gonads is considerably less in the female than in the male, as the ovaries finally settle just below the rim of the true pelvis. Similar to males, a gubernaculum-like structure develops and extends from the inferior pole of the ovary to the subcutaneous fascia of the presumptive labioscrotal folds. It penetrates the abdominal wall through the inguinal canal and carries a slip of peritoneum with it called processus vaginalis. Although the gubernaculum does not shorten like that in males, it still causes the ovaries to descend (by anchoring the ovaries in the pelvis) and places them into a peritoneal fold (the broad ligament of the uterus). This translocation of ovaries occurs during the seventh week, when the gubernaculum becomes attached to the developing Müllerian ducts. The inferior part of the gubernaculum becomes the round ligament of the uterus and attaches the fascia of the labia majora to the uterus. The superior part of it becomes the ligament of the ovary, connecting it to the uterus (Figure 1.3f). As in males, the processus vaginalis of the inguinal canal is usually obliterated, but occasionally it remains patent and can result in an indirect inguinal hernia or hydrocele [Reference Larsen3].
1.2.3.5 Differentiation of External Genitalia
External genitalia development occurs in two stages: hormone-independent growth and hormone-dependent growth.
1.2.3.5.1 Hormone-Independent Growth
This first phase of the development occurs between conception and the seventh to eighth weeks of gestation. It is similar in both genders as the external genitalia are undifferentiated until the ninth week.
This stage is influenced by a cascade of genes including sonic hedgehog (SHH), MNP4, Glia 123 and WT1 gene.
Around the third week, mesenchyme cells migrate from the region of the primitive streak to the perineum, around the cloacal membrane, to form elevated cloacal folds on each side. Anterior to the opening of the urogenital sinus, cloacal folds fuse in midline to form the genital tubercle, which later develops into clitoris in females. At the sixth week, cloacal folds are subdivided into urethral folds anteriorly and anal folds posteriorly (Figure 1.4a).
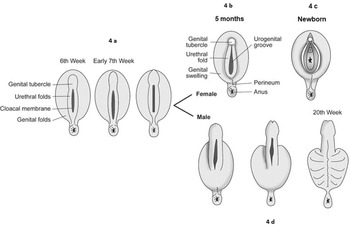
Figure 1.4 External genitalia development. (a) Undifferentiated external genitalia. (b, c) Development of the external genitalia in the female with appearance at 5 months and in the newborn, respectively. (d) Development of the external genitalia in the male.
In the meantime, lateral to the urethral folds, a pair of larger swellings – the labioscrotal folds or genital swellings – become apparent (Figure 1.4b). These join posteriorly, between the urogenital and anal membranes as they separate. The labioscrotal folds later form the labia majora in females and the scrotal sacs in males [Reference Larsen3,Reference Catherine and Rien4,Reference Gearhart, Rink and Mouriquand6,Reference Thomas, Duffy and Rickwood7].
1.2.3.5.2 Hormone-Dependent Growth: Female
Sexual differentiation of external genitalia is hormone-dependent in both sexes. The development of external genitalia is determined by the hormones produced by the gonads, the correct steroid metabolising enzymes pool and the functioning sex hormones receptors. Impairment of any of these factors or environmental influences can cause alterations in the normal pathway.
Female differentiation of the external genitalia begins by the eleventh week and genitalia are defined by the twentieth week of gestation. In female fetuses, oestrogens stimulate the development of the external genitalia:
The genital tubercle elongates only slightly and the phallus bends inferiorly forming the clitoris.
The urethral folds remain unfused and develop into the labia minora.
The genital swellings enlarge and form the labia majora.
The urogenital groove remains open and forms the vestibule in which the urethral meatus, the vaginal orifice and the ostium of the vestibular glands are located (Figure 1.4c).
In addition to oestrogens, the development of external genitalia is promoted by the absence of androgens (dihydrotestosterone). All oestrogens are synthesised from androgen precursors by a unique enzyme called aromatase. Aromatase converts androstenedione, testosterone and 16-hydroxytestosterone into oestrone, oestradiol and oestriol, respectively. Defects in the aromatase gene result in elevated testosterone and virilisation. Other common causes of exposure to androgen include congenital adrenal hyperplasia (CAH). In the process of virilisation, there is trend towards elongation of genital tubercle (enlarged clitoris), fusion of labia majora (scrotalisation of labio-scrotal folds) and fusion of urethral folds (urogenital sinus formation) [Reference Larsen3,Reference Blaschko, Cunha and Baskin5,Reference Thomas, Duffy and Rickwood7,Reference Sajjad9].
1.2.3.6 Embryology and Congenital Anomalies
A good understanding of the embryological development of ovaries, internal and external genitalia will help a clinician in the assessment of anatomical anomalies that can result from a difference in expected development. An improved comprehension of developmental anomalies is a prelude to correct management planning and treatment.
Key Learning Points
The development of internal and external genitalia starts from the same baseline embryological point, and it diverges after the ninth week of gestation into either male or female, depending on the influence of chromosomes, genes and hormones.
Primordial germ cells migrate from the yolk sac to the genital ridges starting at the fifth week of gestation, forming the primitive undifferentiated gonads.
After the tenth week, in the female, the primordial germ cells become primary oocytes and, together with the surrounding epithelium, form the primordial follicle. At this stage the gonad is differentiated into ovary.
From the ninth week of gestation, the absence of anti-Müllerian hormone and the effect of oestrogens released by the ovary cause the differentiation of the paramesonephric ducts into fallopian tubes proximally, uterus, cervix and upper vagina distally.
The external genitalia differentiation is mediated by oestrogens and absence of androgens in female and androgens in males.





