Case description
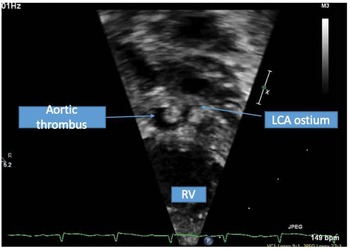
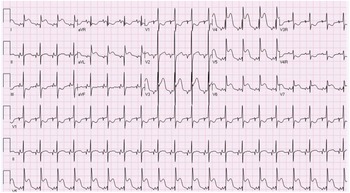
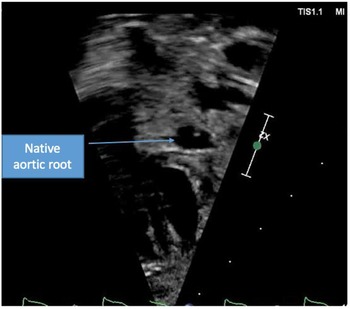
A term male infant was diagnosed postnatally at day 2 after being noted to have tachypnoea which prompted a cardiology workup and was noted to have hypoplastic left heart syndrome with severe mitral and aortic valve stenosis. At 1 week, he underwent Norwood procedure with Sano shunt placement. The Norwood was done with incising down the ascending aorta with side-to-side anastomosis with continuous monofilament sutures with knots on the outside. He had uncomplicated post-operative course and was extubated on post-operative day #5. His initial post-op echo showed unrestrictive atrial septal defect, mild tricuspid regurgitation, patent aortic arch, and Sano shunt with patent Damus–Kaye–Stansel anastomosis with no thrombus noted (Fig 1). On post-operative day #9, he was noted to have sudden onset clinical change after a routine chest physiotherapy and was noted to have sudden onset of bradycardia and hypotension, elevated ST segments with low cerebral and somatic Near Infra-Red Spectroscopy, and elevated blood lactate. A 15 Lead electrocardiogram showed ST elevation (Fig 2). His native aortic root measured around 5 mm and the ascending aorta was 4 mm. The sinotubular junction of the native aortic root measured around 4 mm. An echocardiogram showed a large thrombus (around 5 × 3.5 mm) in his aortic root occluding blood flow into the left main coronary artery (Fig 3a). His labs showed elevated troponins with an initial value of around 14 ng/ml (reference range 0–0.39 ng/ml) which peaked to around 83 ng/ml within 24 hours, with resolution over a week. The patient was intubated, started on low dose epinephrine, and sedated and paralyzed with improved haemodynamics. Haematology was consulted and the patient was started on low dose tissue plasminogen activator at around 0.06 mg/kg/hour, which was titrated to around 0.08 mg/kg/hour based on clot size. Repeat echocardiogram imaging over the next few days revealed decreasing clot size and eventual resolution of thrombus with adequate perfusion of coronary arteries after 72 hours of thrombolysis treatment (Fig 3b).

Figure 1. Short axis of the aortic root with echogenic mass noted in native aortic root consistent with thrombus.
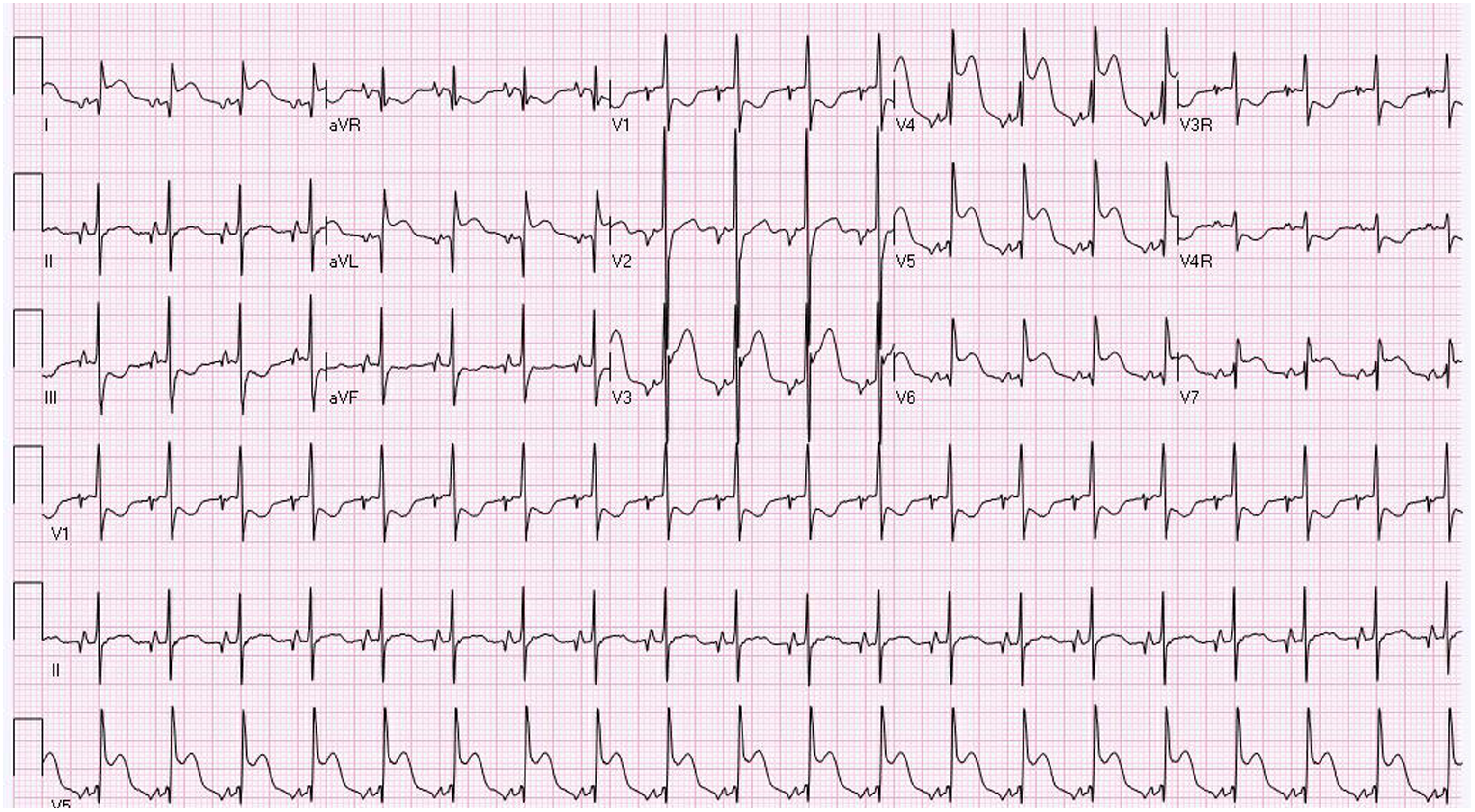
Figure 2. 12 Lead EKG showing evidence of ST elevation in Lead I, AVL, and V4-V6.
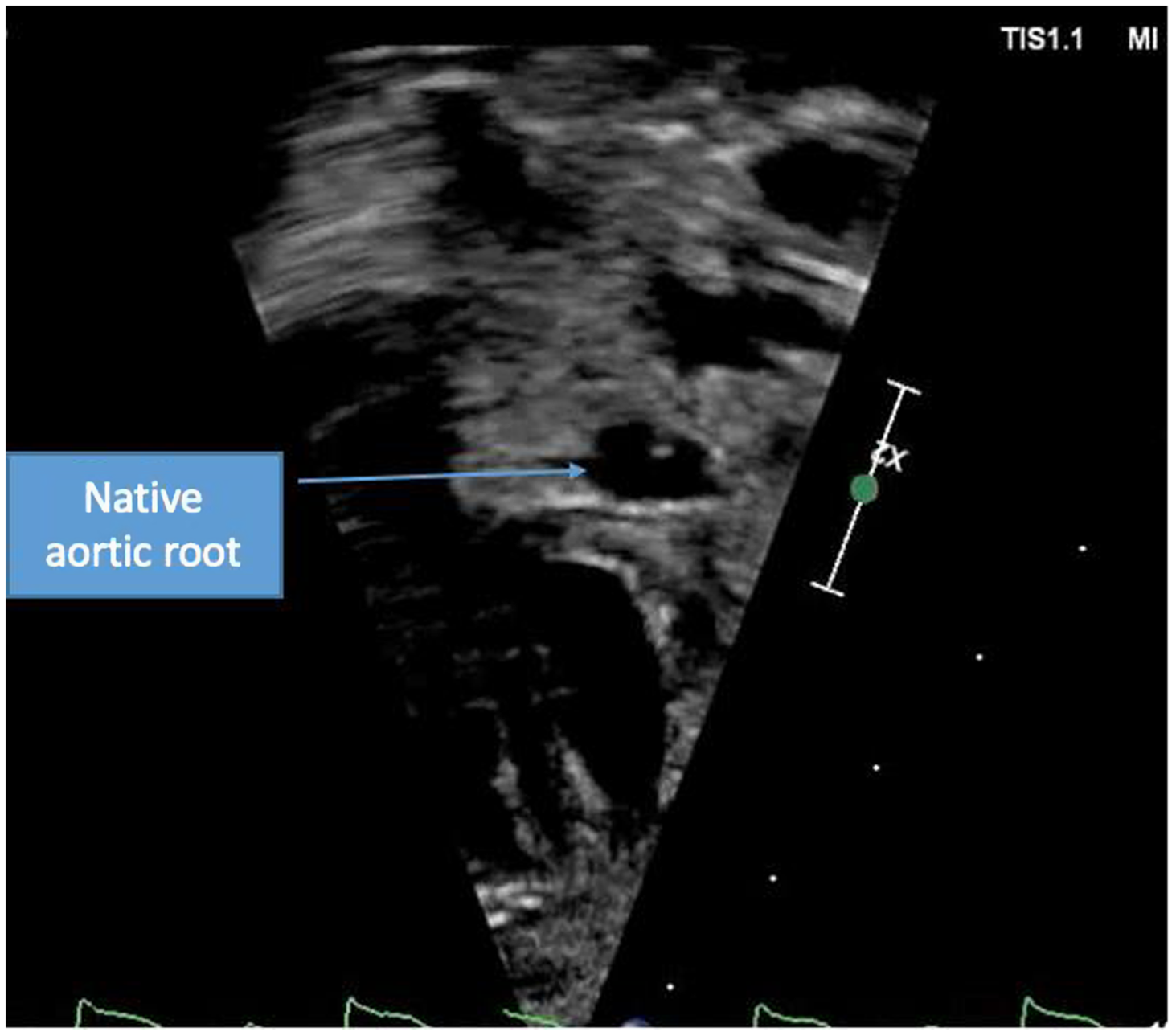
Figure 3. Short axis view of aortic root with resolution of thrombus.
He was discharged on a combination of Lovenox and Plavix. During his work up, he was noted to have additional thrombi in his lower extremity vascular system although his work up revealed no specific aetiologies for his recurrent thrombi.
He is currently around 23 months old and has successfully undergone his Glenn operation and is being maintained on Lovenox for prophylaxis with no recurrence of his aortic thrombus.
Discussion
Neonatal native aortic root thrombus formation is a rare but potentially fatal occurrence if not diagnosed and appropriately managed in patients with hypoplastic left heart syndrome. The factors predisposing children with underlying cardiac conditions to thrombus formation remain unclear. Several cases of thrombus formation in the native aortic root of children with hypoplastic left heart syndrome who have undergone the Norwood procedure have been noted in literature. Reference Janssen, Ohmstede, Liske, Parra, Drinkwater and Ann1 After the Norwood procedure, the native aortic root becomes the pathway channel for coronary blood flow. Reference Owens, Gomez-Fifer and Ensing2 Low flow through this anastomosis might serve as a catalyst for thrombus formation. Reference Mookerjee, Rosenthal and Simpson3 Patency of the native aortic root is critical as it is the solitary source of blood flow for the coronary arteries. Reference Brennan, Rodefeld, Tacy, Reddy and Hanley4 Coronary insufficiency is one of the causes of mortality for hypoplastic left heart syndrome children status post-Norwood procedure. Reference Bartram, Grunenfelder and Van Praagh5 Thrombus formation can present early or also present years later in children with hypoplastic left heart syndrome who have undergone Norwood, Glenn and/or Fontan procedure.
Thrombus formation in the native aortic root in children with hypoplastic left heart syndrome can present as incidental finding or with acute complications postoperatively. Patients can have different clinical presentations and the severity of presentation likely correlates with the degree of coronary ischaemia resulting from aortic thrombus with some patients presenting with only thrombus formation, which might be discovered incidentally without evidence of clinical ischaemia, whereas other patients might present in congestive heart failure secondary to RV dysfunction from myocardial ischaemia. Some children, however, present with cardiac arrest a few days postoperatively with subsequent echocardiography or other studies confirming thrombus formation. Reference Janssen, Ohmstede, Liske, Parra, Drinkwater and Ann1
In our patient, clinical presentation was manifested as ST segment elevation on EKG reflecting ischaemia with elevated troponins. Other previous cases reported in the literature Reference Janssen, Ohmstede, Liske, Parra, Drinkwater and Ann1–Reference Brennan, Rodefeld, Tacy, Reddy and Hanley4,Reference Graham, Shakir and Bradley6,Reference Kosaka, Sakamoto, Takigiku, Yasukochi and Harada7 showed patients in age range of 7 days to 4 years who presented with EKG changes like ST depression, T wave inversion, non-sustained ventricular tachycardia, and complete heart block. Some patients had symptoms of chest pain, and most patients were noted to have elevated biomarkers such as troponin. Some patients with native aortic root thrombus did show evidence of ventricular dysfunction. Most patients were diagnosed with transthoracic echocardiography with one patient diagnosed with cardiac catheterisation. Routine evaluation of the aortic root in all patients who present with new sudden onset RV dysfunction should be done to screen for a possible thrombus in the native aortic root with assessment of coronary flow on echocardiogram. Cardiac catheterisation or advanced imaging should be done in cases with clinical evidence of ischaemia but poor echocardiographic windows or concern for thrombus in coronary arterial system which cannot be assessed with transthoracic echocardiogram.
In children with hypoplastic left heart syndrome, echocardiography is a valuable tool in the assessment of the patency of the native aortic root. Routine evaluation of the native aortic root with echocardiography becomes paramount in the management of these patients as thrombus formation in the native aortic root can become fatal. After clinical confirmation of the thrombus, it is imperative that patients be placed on aggressive anticoagulation therapy. In many cases, including our own patient, anticoagulation therapy can result in the resolution of the thrombus. The fibrinolytic system of neonates has overall decreased activity due to increased plasma levels of plasminogen activator inhibitor and decreased plasminogen activity. Reference Veldman, Nold and Michel-Behnke8 Thus, treating our patient with tissue plasminogen activator allows for increased plasminogen activity and fibrin degradation. In cases where thrombus resolution cannot be achieved, children should be placed on chronic anticoagulation with low molecular weight heparin. Thrombolytic therapy is another potential management of a thrombus. However, there is an increased risk of distal embolisation due to fragments of the thrombus dislodging in, and therefore occluding, the coronary arteries. Given the risks associated with systemic tissue plasminogen activator especially in the presence of additional thrombi which are common in these patients, risk benefit ratio must be assessed before initiating treatment in these patients. Therefore, while patients can be managed with thrombolytic therapy, they must remain on anticoagulation to prevent further thrombus formation. It is important to note, however, that anticoagulation with heparin does not guarantee complete thromboprophylaxis, as recurrent thrombus progression in the native aortic root has been noted with therapy. In such cases, the thrombus combined with deteriorating right ventricular function can give way to cerebrovascular embolic events. Reference Mitchell, Berman, McConnell and Buber9
We recommend initiation of treatment with heparin typically low molecular weight heparin only in cases where there is evidence of thrombus but with no evidence of ischaemia with close follow-up and monitoring to assess for evidence of clinical ischaemia. However, when there is a documented thrombus with evidence of ischaemia, treatment with tissue plasminogen activator should be initiated systemically. It is difficult to recommend local tissue plasminogen activator in all patients given the concerns about the small DKS in these patients with hypoplastic native aortic root. Surgical removal should be used as a last resort in thrombi confined to the aortic root which do not resolve with thromboprophylaxis.
Conclusion
Routine echocardiography is an essential part of management of hypoplastic left heart syndrome children after Norwood and/or Fontan procedure. We recommend a routine evaluation to ensure patency of the native aortic root as thromboembolic events can be clinically symptomatic or silent. Myocardial ischaemia in these patients should be confirmed by a combination of EKG, Echo, CT, cardiac catheteriszation, and laboratory markers. Cardiac catheterisation should be reserved for patients not improving or with unclear location of thrombus. In patients with clinical signs of ischaemia, treatment with tissue plasminogen activator should be initiated. Clinically silent thrombus should be treated with anticoagulation. In addition, patients should be placed on chronic anticoagulation immediately post-operative and after the diagnosis of a thrombus formation even if resolution is achieved due to risk of recurrence.
Financial support
This research did not receive any grants from any funding agency or non-profit sectors.
Conflicts of interest
None.