INTRODUCTION
Leptospirosis is presumed to be the most widespread zoonosis in the world. It can be transmitted, in both man and animals, by direct or indirect contact with infected materials. Infected animals excrete leptospires in their urine, which constitute the primary route for further transmission of the infection through contact with contaminated water and soil or urine itself [Reference Adler and de la Pena Moctezuma1]. Dogs are significant reservoir for human infection and may be an important source of outbreaks [Reference Brown and Prescott2].
Canine leptospirosis occurs worldwide and was recognized as a disease of dogs in 1899 before it was recognized in any other animal species, including humans [Reference Andre-Fontaine3, Reference Faine4]. Traditionally, canine leptospirosis has mainly been associated with serovars Canicola and Icterohaemorrhagiae, which are from two different serogroups within Leptospira interrogans species. Leptospires are known to be highly pathogenic in dogs and four syndromes have been identified in infected dogs: icteric, haemorrhagic, uraemic and reproductive [Reference Adler and de la Pena Moctezuma1]. None of them are exclusively associated with one serovar. Detection of antibodies against the surface antigens of the various leptospiral serovars by the microscopic agglutination test (MAT) is the most common test for leptospirosis [Reference Adler and de la Pena Moctezuma1].
Recent publications from around the world, including Europe, have highlighted the re-emergence of canine leptospirosis and its zoonotic potential. A recent study in Ireland indicated that 7% of domestic dogs were shedding leptospires in their urine [Reference Rojas5] and in Germany an increase in the number of human cases has in part been attributed to resurgence of canine leptospirosis [Reference Jansen6]. Leptospirosis has also appeared as a sporadic human health problem in Greece and severe fatal syndrome occurs every year [Reference Bisias7–Reference Sion9].
Vaccines for the protection of dogs against L. interrogans infection have been available in Europe for about 50 years [Reference Jull and Heath10]. Traditionally these included serovars Canicola and Icterohaemorrhagiae. Since vaccine immunity is primarily serovar-specific, infection with serovars other than Canicola and Icterohaemorrhagiae infection by other serovars cannot be controlled by the currently available vaccines. Reports from some parts of Europe indicate an altered epidemiological situation and there have been calls for an expansion of the number of Leptospira serovars included in vaccines to reflect the most prevalent serovars currently found in dogs [Reference Gerlach and Stephan11, Reference Geisen, Stengel and Hartmann12]. Changes in the epidemiology of canine leptospirosis in North America have resulted in the inclusion of serovars Grippotyphosa and Pomona in bacterins available there. In Europe, vaccine manufacturers are actively reviewing the strains of Leptospira that should be included in dog vaccines [Reference Davies13] and whether there is common ground between European and North American requirements. A review of the evidence for such changes found a lack of recent published information on canine leptospirosis in many parts of Europe [Reference Ellis14]. Vaccine manufacturers prefer to seek licences on a European Union (EU)-wide basis rather on a country-by-country basis. Serovars appropriate for inclusion in dog vaccines for countries where there is recent prevalence data, may not be appropriate for all member states, Greece was selected for the present study because it is one of the EU member states for which there is limited information [Reference Burriel, Dalley and Woodward15]. The purpose of this study was to investigate the seroprevalence of potential vaccine candidate strains in dogs in northern Greece in order to evaluate whether there is supporting evidence for the inclusion of serovars Grippotyphosa, Pomona and Bratislava in dog vaccines. In addition, serovar Bratislava has been included in this survey as it was identified as another likely European vaccine candidate [Reference Ellis14]. European strains of the Pomona serogroup (Mozdok and Altoduoro) were included for the first time as they are more appropriate to European studies than serovar Pomona [Reference Ellis14, Reference Paiva-Cardoso16].
MATERIALS AND METHODS
Serum samples from 855 dogs (469 females, 386 males) were collected by the Companion Animal Clinic, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki between 2006 and 2010. Most of the sera (697, 81·5%) came from dogs which had been vaccinated against serovars Icterohaemorrhagiae and Canicola. The sera were stored frozen at the clinic prior to shipping to the Leptospira laboratory in Belfast accompanied by the relevant clinical veterinary information.
All samples were tested for leptospiral antibodies by MAT [Reference Wolff17] using live antigens of L. interrogans serovar Bratislava, L. kirschneri serovar Grippotyphosa and three antigens representing the Pomona serogroup of leptospires – L. interrogans serovar Pomona, L. kirschneri serovar Mozdok, and the putative new serovar Altodouro (strain Rim 139) [Reference Paiva-Cardoso16].
Each antigen was grown in 10 ml volume of EMJH medium, incubated at 28 °C. After 6–10 days' growth (depending on the antigen) antigens were adjusted to 1–2 × 108 cells/ml by cell count using a counting chamber.
The sera were initially scanned for antibodies to the five antigens at a final dilution of 1:30 and 1:300 being incubated for 2 h at 28 °C. Where there was evidence of agglutination the relevant sera were end-point tested using dilution patterns proposed by Ellis & Michna [Reference Ellis and Michna18] from 1:10 to 1:30000.
The serological titre for any serovar was considered to be the highest dilution where there was sufficient antibody present to agglutinate ⩾50% of the antigen with the exception of the 1/10 dilution where there was required to be 75% agglutination of the antigen.
Dogs were deemed to have been exposed to infection when titres of 1:10 or greater were detected and seroprevalence in this study was defined as the proportion of submitted samples with positive MAT results. For any positive sample, the infecting serovar was assumed to be that which demonstrated the highest titre and the lower titres were considered to be cross-reactions.
All statistical analyses were performed on data from the seropositive dogs and the serovars to which they were likely exposed. Risk factors for the presence of Leptospira antibodies were assessed by nominal logistic regression analysis. The strength of association between seropositivity and other variables, were estimated by the calculation of odds ratio (OR), and OR with a lower 95% confidence interval (CI) >1 was taken to indicate a significant association [Reference Bland and Altman19]. There were several variables analysed, such as age, origin (rural/urban) and sex.
RESULTS
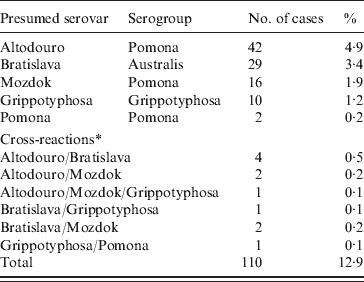
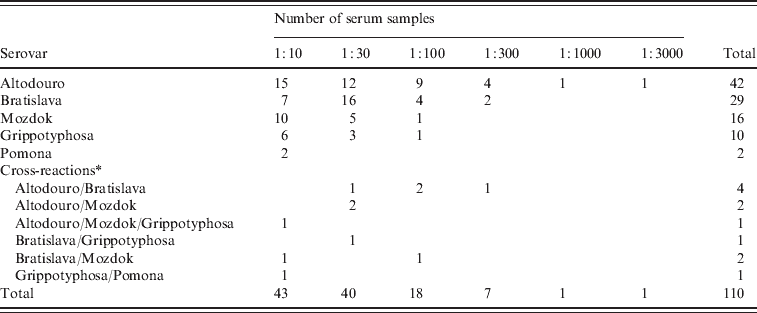
The results of serological tests of 855 dogs are reported in Tables 1 and 2. A total of 110 (12·9%) dogs had antibody titres of 1:10 or greater to at least one of five serovars used in the study. The majority (91·8%) of positive samples demonstrated low titres (⩽1:100). The highest titres ⩾1:1000 were determined for two (1·8%) samples with serovar Altodouro.
Table 1. Positive results of the microscopic agglutination test by presumed serovar and serogroup of 855 dogs
* Cross-reactions at equal titres.
Table 2. Distribution of antibody titres to five Leptospira serovars in 110 dogs
* Cross-reactions at equal titres.
Titres to the Pomona serogroup were the most common with antibodies to serovar Altodouro being present in 4·9% of sera with titres ranging from 1:10 to 1:3000. Antibodies to the other Pomona serogroup strains were much less common with seroprevalences for Mozdok (titre range 1:10 to 1:100) and Pomona of 1·9% and 0·2%, respectively. Only 1:10 titres were detected to serovar Pomona. Serovar Bratislava was the second most prevalent serovar (3·4%) with titres ranging from 1:10 to 1:300. The serological prevalence for Grippotyphosa was 1·2%, with titres from 1:10 to 1:100. Multiple reactions, at equal titres, were observed in 1·3% of the sera tested.
Most (88·2%) positive dogs were reported to be clinically healthy. No significant association was found between a diagnosis of leptospirosis and the presence of antibodies to serovars Bratislava, Mozdok, Pomona and Grippotyphosa. A significant association was observed in relation to serovar Altodouro. Of positive dogs, only 12·2% of vaccinated dogs and 15·8% of unvaccinated dogs had exposure to at least one serovar (OR 0·739, 95% CI 0·456–1·199).
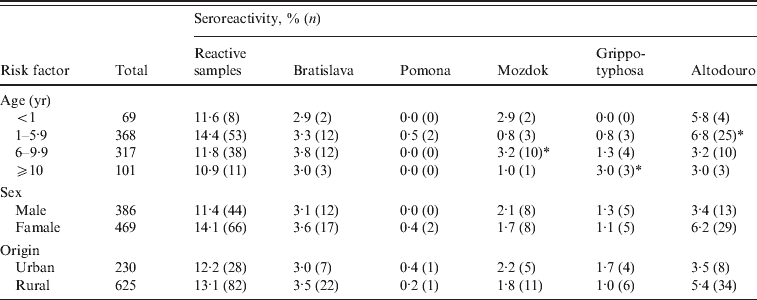
Seroprevalences for all positive dogs were similar in those living in rural areas (13·1%) compared to those living in urban regions (12·2%) with the exception of serovar Altodouro where a greater, but not significant, difference was observed – 5·4% for rural and 3·5% for urban dogs (OR 1·596, 95% CI 0·728–3·502).
When seroprevalences for all five serovars were considered together with respect to age, a higher rate was observed in dogs aged between 1 and 5·9 years. At this age the dogs appeared to be more likely to have antibodies to serovar Altodouro (6·8%; OR 2·015, 95% CI 1·071–3·790). Older dogs aged >10 years were more likely to have antibodies to Grippotyphosa (3·0%; OR 3·267, 95% CI 0·831–12·841), the age category 6–9·9 years was significant for antibodies to serovar Mozdok (3·2%; OR 2·888, 95% CI 1·040–8·024). No statistical difference was detected regarding the other serovars (Table 3).
Table 3. Leptospira seroprevalence by age, sex and origin
* Results statistically significant.
There was a higher prevalence of antibodies to all serovars in females (14·1%) than in males (11·4%) (OR 1·090, 95% CI 0·689–1·723); however, sex was not significantly associated with exposure of leptospirosis. The prevalence of antibodies to serovar Altodouro was higher in females (6·2%) than males (3·4%), but no significant difference was found between them (OR 1·596, 95% CI 0·728–3·502).
DISCUSSION
There are two points which must be considered when assessing the value of including new serovars in a dog vaccine. First, whether there is evidence of the serovar causing clinical disease in dogs and second, whether dogs are being infected by that serovar and therefore likely to be a risk factor for human leptospirosis or leptospirosis in other domestic animals.
This study has shown that dogs in northern Greece are being exposed to infection by strains of the Pomona serogroup, serovar Bratislava and occasionally serovar Grippotyphosa. Whether dogs are persistently infected with any of these remains to be determined as this can only be assessed by renal culture which was not part of this study.
The very low seroprevalence to serovar Pomona is consistent with the very low prevalence to this serovar found across most of Europe, with the exception of Romania [Reference Cabrevetanabreve and Fodor20]. This low seroprevalence in Europe led Ellis [Reference Ellis14] to conclude that there was no case for the inclusion of serovar Pomona in European dog vaccines. He suggested there was a need for studies in which serovar Mozdok, rather than serovar Pomona, was included as a test antigen because this serovar is present in a number of European rodent species to which dogs would have exposure [the striped field mouse (Apodemus agrarius), the great white-toothed shrew (Crocidura russula) and the Algerian mouse (Mus spretus)]. While seroprevalences to serovar Mozdok were also very low in this study, the data from the inclusion of the recently isolated serovar Altodouro as a test antigen questions the conclusion of Ellis [Reference Ellis14]. In our study, one in 20 dogs had evidence of exposure to this serovar and there was statistical evidence indicating that serovar Altodouro was associated with clinical disease in dogs. There would therefore be a case for the inclusion of a Pomona serogroup strain in Greek dog vaccines. Whether serovar Pomona, which is present in vaccines in the USA, may be appropriate would depend on whether such vaccines could be shown to cross-protect against serovar Altodouro.
Serovar Altodouro has only been isolated very recently – from house mice (Mus musculus) in northern Portugal [Reference Paiva-Cardoso16]. The finding that it is a much more sensitive antigen for the exposure of Greek dogs to Pomona serogroup infection may have important implications for what is known about these infections in other European countries and whether there is a need for the inclusion of a Pomona serogroup strain in European dog vaccines. More canine seroprevalence studies across Europe using this antigen are now required.
Seroprevalence data for the last 20 years indicates widespread exposure of dogs to serovar Bratislava infection in Europe [Reference Gerlach and Stephan11, Reference Geisen, Stengel and Hartmann12, Reference Rey21–Reference Scanziani25]. There are a number of known maintenance hosts for this serovar – hedgehogs, pigs and horses [Reference Ellis26, Reference Ellis27] and probably dogs [Reference Brem, Kopp and Meyer22]. Serovar Bratislava was the second most common serovar to which dogs were found to be exposed in this study. This is consistent with the findings of Burriel et al. [Reference Burriel, Dalley and Woodward15]. They also showed that serovar Bratislava may infect a range of other domestic animals in Greece.
A case for the inclusion of serovar Grippotyphosa in dog vaccines has been made by Ellis [Reference Ellis14] but the findings in that study would not support the need for it in Greece. This may be because the ranges of the known carrier rodent hosts [the common vole (Microtus arvalis), the root vole (Microtus oeconomus), the muskrat (Ondatra zibethicus), and the common hamster (Cricetus cricetus)] of serovar Grippotyphosa do not extend to Greece.
There was no obvious significance regarding any of the risk factors (age, sex, urban/rural origin) and exposure to any of the five Leptospira serovars included in the study. Only serovar Altodouro was found to be statistically significant when associated with one risk factor – age. The finding in the current study is that seroreactivities to Altodouro were of much higher incidence in young dogs (0–5·9 years). It suggests that the younger animals play a particularly important part in the epidemiology of infection. It confirms other published results [Reference Claus28, Reference Hartman29] which showed that younger dogs are more severely affected by leptospirosis than older dogs. However, a different situation was observed for serovars Grippotyphosa and Mozdok for which the risk of infection was greater in older (>10 years) and middle-aged (6–9·9 years) dogs, respectively. Those dogs may be more active outside their normal home environment than young dogs, increasing potential exposure to those two serovars, mainly maintained by wild-life hosts.
Regarding differences between rural and urban dogs, in spite to the observation that reactive samples were greater for rural than for urban dogs, no significant differences were found between the two populations. Moreover, no significant statistical differences in seroprevalence were found between sexes, although predisposition for leptospiral infection in males has been previously suggested [Reference Van den Broek23].
This study has shown that, in northern Greece, dogs are being exposed to infection by strains of the Pomona serogroup, serovar Bratislava and occasionally serovar Grippotyphosa and that an association can be shown between infection with serovar Altodouro (Pomona serogroup) and clinical disease in dogs. Whether dogs are persistently infected by any of these can only be assessed by a renal culture study.
These findings support the view that inclusion of a Pomona serogroup strain and serovar Bratislava should be considered for dog vaccines in Greece and may indicate the need for proportional studies across Europe.
An important aspect of the study is its zoonotic implications. Since humans and dogs in part share their environment, humans are also basically exposed [Reference Brown and Prescott2, Reference Houwers30]. Dogs, as a significant reservoir for human infection, were seen to be an important source of outbreaks [Reference Levett31]. For example, urine shedding by infected dogs played a major role in the outbreaks of human leptospirosis in Nicaragua in 1995 [Reference Trevejo32]. Dogs were also recognized as important risk factor for human leptospirosis in Barbados [Reference Douglin33] and a potential reservoir for this disease in Germany [Reference Jansen6].
To control leptospirosis in dogs, vaccination is essential and considered to be the front-line defence against the disease. Its purpose, besides reducing the severity of the clinical signs, is to prevent renal infection and urine shedding. This is important in order to limit the zoonotic risk and transmission of the pathogens between animal populations [Reference Bisias7, Reference Broughton and Scarnell34–Reference Schreiber36].
ACKNOWLEDGEMENTS
This work was financially supported by Pfizer Limited, Sandwich, UK (Study no: 9Z60W-16-09-246). The authors are grateful to Heather Milligan and Fiona McBride for their technical support in the laboratory.
DECLARATION OF INTEREST
None.







