Streptococcus pneumoniae is the most common bacterial aetiology of community-acquired pneumonia in all ages, and can cause outbreaks in closed settings [Reference Heymann1, Reference Balicer2]. Transmission is by droplet spread, and person-to-person transmission is common [Reference Heymann1]. Carriage is an important step for pneumococcal infection and illness [Reference Bogaert, Groot and Hemans3]. Most data on pneumococcal transmission derive from studies in children and sometimes their caregivers [Reference Hussain4, Reference Greenberg5], but transmission patterns in healthy young adults are less well known. A recent outbreak of severe pneumococcal illness in an Israeli Army training base [Reference Balicer2] led to us to undertake a study to characterize pneumococcal carriage prevalence and risk factors in healthy young adults upon their recruitment to military service.
The study was approved by the Medical Corps Ethics Board of the Israeli Defense Force (IDF). This cross-sectional study was conducted on three different recruitment days, in March, July and November 2007, in consecutive groups of male recruits to one IDF training base, before mixing and training in confined settings, reflecting carriage acquired before entering the base. This study was performed before the introduction of routine pneumococcal childhood vaccination in Israel on July 2009. All recruits were asked to participate in the study and, after providing informed consent, completed a questionnaire and allowed oropharyngeal and nasopharyngeal sampling for pneumococcal culture. The questionnaires were written in clear and simple Hebrew and included questions on sociodemographic variables, morbidity and health behaviour, reflecting the recruit's status before entering the base. Questionnaire instructions were given at each visit by the study physician in the same manner. Pre-recorded demographic data, such as mother's origin and socioeconomic status of residential area were accessed from the IDF computerized database. The sampling was conducted by a trained team of healthcare workers according to a defined protocol. Samples were inoculated onto a transport medium, cultured within 2–18 h and processed for identification and serotyping [Reference Dagan6]. Statistical analysis was performed with SAS software, version 9.1.3 (SAS Inc., USA). All risk factors with P<0·05 found in the univariate analysis, in addition to one previously known risk factor (smoking), were included in the multivariate logistic regression analysis evaluating pneumococcal carriage risk factors. Ninety-five percent confidence intervals (95% CI) were calculated for carriage prevalence.
Of 1340 male recruits invited to participate in the study, 776 (57·9%) agreed to participate and 742/776 (95·6%) underwent proper sampling. None of the recruits had a known underlying medical condition, as that was an exclusion criterion for recruitment to this base. Mean age was 19·2 years (range 18·1–24·3 years); 92% of participants were aged 18–20 years.
Streptococcus pneumoniae was isolated from 49 study participants which comprised 6·6% (95% CI 4·8–8·4) of the 742 subjects. Three percent (95% CI 1·9–4·5), 2·4% (95% CI 1·4–3·8) and 1·2% (95% CI 0·6–2·3) of the subjects carried S. pneumoniae in the nasopharynx only, oropharynx only, or both, respectively. In our study 64% of carriers had a positive nasopharyngeal culture, and 55% of carriers had a positive oropharyngeal culture, of whom 67% had negative nasopharyngeal culture. Kappa agreement between nasopharyngeal and oropharyngeal results was 0·28 (95% CI 0·12–0·44, P<0·001). Univariate analysis for pneumococcal carriage by sociodemographic and health behaviour characteristics are presented in Table 1. Of 352 (48·1%) subjects who reported sharing a drinking glass/bottle ‘always/usually’ (frequent sharing group), 33 (9·4%) carried S. pneumoniae, compared with 16 (4·2%) of the 380 subjects who reported sharing glass/bottle with others ‘half of the time/occasionally/never’ with odds ratio (OR) of 2·35 (95% CI 1·27–4·36, P=0·005). There was a significant correlation between carriage prevalence and frequency of sharing a drinking glass for each category: always (15/151, 9·9%), usually (18/201, 9·0%), half of the time (9/194, 4·6%), occasionally (6/151, 4·0%), never (1/35, 2·9%); (OR 1·43, 95% CI 1·10–1·88, P=0·007). Carriage rates by anatomical sampling site (nasopharynx only, oropharynx only, or both) in the frequent glass/bottle-sharing group compared to the non-frequent sharing group were 4·6% vs. 1·6%, 3·1% vs. 1·8%, and 1·7% vs. 0·8%, respectively. The frequent sharing group had higher carriage prevalence both in nasopharynx and oropharynx. However, when comparing the frequent and non-frequent sharing groups per isolation site, the difference reached statistical significance for nasopharynx (6·3% vs. 2·4%, OR 2·75, P=0·01, Fisher's exact test) but not for oropharynx (4·8% vs. 2·6%, OR 1·88, P=0·12, Fisher's exact test). Other hygiene practices (i.e. washing hands after toilet use or before meals) were not associated with carriage. Multivariate logistic regression analysis, controlling also for birthplace of mother, age and smoking is shown in Table 2. Of 32 (4·4%) subjects who reported taking antibiotic treatment up to 1 month prior to study recruitment, none carried S. pneumoniae. However, there was not a significant difference in carriage prevalence in these subjects compared to the rest (P=0·12). Controlling for antibiotic use in an alternative multivariate analysis did not have an impact on the results of the model and due to statistical considerations this variable was not included in the model.
Table 1. Univariate analysis for pneumococcal carriage by sociodemographic and health behaviour characteristics of 742 study subjects
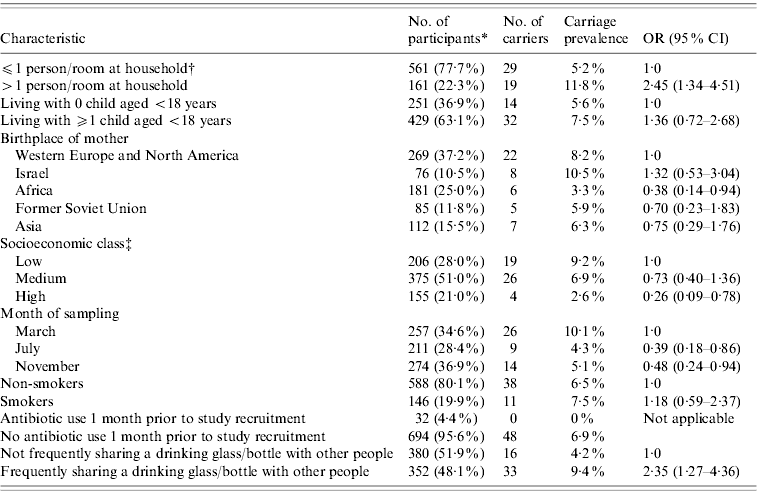
OR, Odds ratio; CI, confidence interval.
* Values within parentheses are the percentage of participants from those with known characteristic.
† Calculated by dividing the number of persons living with the participant in the household (including himself) divided by the number of rooms in the household (excluding living room), based on answers to the questionnaires.
‡ According to place of residence, based on Central Bureau of Statistics 2003 classification of municipalities by the socioeconomic characteristics of the population (Jerusalem, Israel: Central Bureau of Statistics, 2003).
Table 2. Multivariate logistic regression analysis for pneumococcal carriageFootnote *

OR, Odds ratio; CI, confidence interval.
* Controlling for birthplace of mother, age and smoking (688 subjects included in the analysis).
There were no other variables with P value between 0·05 and 0·2 in the univariate analysis. Frequent glass/bottle sharing remained associated with pneumococcal carriage (P=0·008, OR 2·40, 95% CI 1·26–4·59). Other independent significant risk factors included having >1 person/room at the household, and March season/cohort compared to July. High compared to low socioeconomic status as judged by residential area was not a significant risk factor in the multivariate model (P=0·08, OR 0·37, 95% CI 0·12–1·19). Since a correlation was found between having >1 person/room at the household and socioeconomic status of residential area (Pearson correlation coefficient=0·18, P<0·0001), the multivariate analysis was repeated without this variable. In this case, socioeconomic status as judged by residential area was found significant (P=0·04, OR 0·29, 95% CI 0·09–0·91) for high vs. low status. Interestingly, there was no association between frequent glass/bottle sharing and socioeconomic status (P=0·72). Public health impact was estimated by calculating population attributable fraction (PAF) for the preventable risk factors [Reference Abramson7]. This calculation is based on OR and proportion of the population with a given risk factor and reflects the excess carriage proportion of all carriage in the population attributed to the risk factor, if the association is casual. The PAF estimate for frequent glass/bottle sharing was 40·2% (95% CI 11·1–63·3).
In total, 55/58 (95%) pneumococcal isolates were serotyped and 22 distinct serotypes were found, plus untypable isolates. The most common serotypes found were 3, 6A, 19F and 34.
Upon recruitment, prior to mixing and training in confined settings, one of every 16 subjects carried S. pneumoniae. In adults, unlike children, oropharyngeal carriage contributes significantly to pneumococcal carriage detection, suggesting that saliva could be an important vector in adults [Reference Greenberg8]. Sharing drinking utensils is a common practice in young adults in Israel, as 48% of participants were frequently sharing a drinking glass/bottle before recruitment. Our study proved frequent sharing of a drinking glass/bottle to be a common, strong and independent risk factor for pneumococcal carriage. These results are unlikely to be associated with general hygiene practices, since no correlation was found between hand-washing frequency and pneumococcal carriage and not confounded by other known risk factors such as smoking or crowding as those were controlled for. Pneumococci were first isolated in 1881 through inoculation of rabbits with human saliva by Pasteur and Sternberg and additional studies have reiterated these findings of pneumococci existing in the saliva [Reference Klein, Feigin, Cherry, Demmler and Kaplan9, Reference Ross10]. However, pneumococcal transmission through saliva has not been previously investigated. Our findings strongly suggest that saliva may transmit pneumococci in adults.
The results suggest seasonality in pneumococcal carriage but only three time-points were examined in three different groups, therefore interpretation of seasonality should be done cautiously. The results validate previous finding of crowding at home as a risk factor for pneumococcal carriage and suggest this is at least part of the explanation for higher carriage in low socioeconomic groups.
Our study has several limitations. First, the sample size was large enough for an analysis of common and strong risk factors but we could not rule out other possible risk factors such as smoking. Second, only 55% of the potential subjects agreed to participate in the study. However, a comparison of available sociodemographic characteristics did not reveal major differences between the groups (data not shown) and there is no reason to suspect different associations between risk factors and carriage in volunteers and non-volunteers. Third, frequency of glass/bottle sharing was based on self-report. However, participants and researchers were not aware of carriage status at the time of data collection. Fourth, although we controlled for several confounders, we did not control for other known confounders for pneumococcal carriage, such as Staphylococcus aureus carriage or important confounders shown in studies of other pathogens, such as social behaviours: intimate kissing or attendance at pubs/bars [Reference Regev-Yochay11, Reference MacLennan12]. Indeed, this study, as well as other recent studies, teaches us that oral behaviours such as sharing a drinking glass should be included in further studies or outbreak investigation of risk factors for saliva-transmitted pathogens, such as pneumococci.
Based on the present study, pneumococcal transmission through saliva by sharing of a drinking glass/bottle is highly plausible in young adults. To the best of our knowledge this has not been previously reported. These findings are used in health education campaigns and outbreak control in the IDF and may be important for health professionals who deal with epidemiology, prevention or control of pneumococci and pneumococcal-related diseases as well as other pathogens.
ACKNOWLEDGEMENTS
We thank Yulia Alexandrovsky, Raid Kayouf and Army Health Branch staff for patiently collecting information and samples; Alon Zahavi and Hanan Goldberg and base clinic staff for setting and coordinating the samplings at the base; Ruhama Ambar, Nurith Porat and laboratory staff for laboratory work management; Françoise Andrijasevic for her assistance with English editing. This study was part of Dr Levine's requirements for obtaining a MPH degree at the School of Public Health, Tel Aviv University. This study was supported by a research grant from the IDF Medical Corps and the Israeli MOD.
DECLARATION OF INTEREST
None.






