The increasing prevalence of diabetes remains an important global public health problem( Reference Shaw, Sicree and Zimmet 1 ). Globally, the excess mortality from diabetes was estimated as 4 million adult deaths in 2010, which accounted for 6·8 % of global deaths( Reference van Dieren, Beulens and van der Schouw 2 ). As research has shown that lifestyle factors, including diet, are key modifiable risk factors for the prevention of type 2 diabetes( Reference Bazzano, Serdula and Liu 3 ), optimizing the balance of macronutrients in the diet is an important protective influence in reducing the risk of developing type 2 diabetes.
Current evidence on the association between dietary macronutrients and type 2 diabetes has been conflicting and not yet fully elucidated. Observational studies that examined the association between dietary carbohydrates and type 2 diabetes have provided mixed results( Reference Meyer, Kushi and Jacobs 4 , Reference Villegas, Liu and Gao 5 ) and results of the few epidemiological studies that examined protein intake and type 2 diabetes risk are inconsistent( Reference Sluijs, Beulens and van der A 6 – Reference Wang, de Koning and Kanaya 8 ). Fatty acid consumption has received a lot of attention given their properties that relate to both health outcomes and disease. Such effects include influencing glucose metabolism by altering cell membrane function, enzyme activity, insulin signalling and gene expression( Reference Riserus, Willett and Hu 9 ). Despite the metabolic impact of fatty acids in type 2 diabetes, research investigating dietary fat intake and type 2 diabetes is sparse( Reference Meyer, Kushi and Jacobs 10 , Reference Salmeron, Hu and Manson 11 ).
One putative explanation for these conflicting results may be the diet variation between countries. Therefore, from a preventive perspective, it would be useful to examine the association of macronutrient intake with type 2 diabetes risk in various populations. Considering that information on macronutrient intake and type 2 diabetes risk in Australian populations is scarce, an additional cohort analysis within the Australian population may provide important evidence on this association. The purpose of the present analysis was to examine the association between dietary macronutrients and the risk of developing type 2 diabetes over 6 years of follow-up, with particular emphasis on dietary fatty acids, in a nationally representative cohort of middle-aged Australian women.
Materials and methods
The Australian Longitudinal Study on Women's Health (ALSWH) commenced in 1996 when approximately 40 000 women from all states and territories completed a mailed questionnaire investigating factors affecting the health and well-being of three age cohorts of women: young (18–23 years), middle (45–50 years) and old (70–75 years). Women were randomly selected from the national health insurance database (Medicare) that includes all permanent residents of Australia with an over-representation of women from rural and remote areas. The study collects self-reported data using mailed surveys at 2- to 3-year intervals. Further details of the cohort profile have been reported elsewhere( Reference Lee, Dobson and Brown 12 ).
A total of 9101 middle-aged women who completed the FFQ in survey 3 (2001) were considered for the present analysis. Women who reported a daily energy intake of less than 3347 kJ (800 kcal) or above 25 104 kJ (6000 kcal; n 291) or who had a history of diabetes (n 440) were excluded from the analysis, leaving 8370 women for the final study analyses.
The study was conducted according to the ethical guidelines of the University of Newcastle and the University of Queensland Human Research Ethics Committees.
Dietary intake assessment
Diet was assessed using the Dietary Questionnaire for Epidemiological Studies (DQES) version 2, which was developed for use with Australian adults( Reference Ireland P Jolley and Giles 13 ). This FFQ was administered at survey 3 and asked respondents to report their usual consumption of seventy-four foods and six alcoholic beverages. Ten possible consumption responses, ranging from ‘never’ to ‘up to three or four times per day’, were given for each food. Portion photographs of vegetables, potatoes, meat and casserole dishes were provided to assist with quantifying the amounts of food consumed. Additional questions were asked about the number of servings and type of fruit, vegetables, bread, dairy products, eggs, fat spreads and sugar consumed. Nutrient intakes were computed from NUTTAB 1995, a national government food composition database of Australian foods( 14 ), using software developed by the Cancer Council of Victoria. The validation of the FFQ was previously assessed for sixty-three women of childbearing age against 7 d food diaries and was found to be a valid instrument for assessing dietary intake. Energy-adjusted correlation coefficients for macronutrient intakes ranged from 0·30 (protein) to 0·78 (carbohydrate), but this did not include fatty acids composition such as n-3 PUFA and n-6 PUFA as data were not available( Reference Hodge, Patterson and Brown 15 ).
Ascertainment of type 2 diabetes
The occurrence of type 2 diabetes was self-reported by participants. At each survey women were asked if a doctor had told them that they had type 2 diabetes. At survey 1 they were asked whether they had ever had a diagnosis of type 2 diabetes and then at surveys 2, 3, 4 and 5 they were asked whether they had been diagnosed with type 2 diabetes in the time period that had elapsed since the previous survey. Incidence of type 2 diabetes at each survey was defined as the proportion of these women who reported at each survey that they had been diagnosed with type 2 diabetes since completing the previous surveys.
Self-reports of type 2 diabetes were validated in middle and old age cohorts by linking to Medicare (MBS) and Pharmaceutical Benefits Scheme (PBS) databases for the years 2002–2005. A total of 6921 middle-aged women completed survey 4 and consented to the release of Medicare data. Among these women, 388 (6 %) of the middle-aged women reported they had diabetes on at least one survey. Of these women with diabetes, ninety (23 %) of the middle-aged women had a Medicare item for annual cycle of care for diabetes (ACC), which includes a glycated haemoglobin (HbA1c) test at some time during the years 2002–2005. A further 184 (47 %) of the middle-aged women had HbA1c but not the full ACC. The remainder of the women reporting diabetes (30 %) had no record of either of these Medicare items. Therefore, diabetes reporting was confirmed in 70 % of self-reported diabetics in this population( Reference Lowe, Byles and Dolja-Gore 16 ).
Other measurements
The baseline questionnaire included questions on known or suspected type 2 diabetes risk factors which are potentially associated with quality of food intake. Area of residence was categorized based on an index of distance to the nearest urban centre as: urban (capital city or other metropolitan centres); rural (large rural centre, small rural centre or other rural); or remote areas( 17 ). Education was categorized as: less than year 10 or equivalent (schooling to age 15/16 years); year 12 or equivalent (schooling to age 17/18 years); trade/certificate; or university degree. Physical activity scores were derived from self-reported frequency and intensity of leisure-time physical activity. These questions were revised from those developed for monitoring and evaluation of the national Active Australia campaign( Reference Armstrong, Bauman and Davis 18 ). Physical activity was categorized as: none; low; moderate; or high. Cigarette smoking status was defined as: never smoked; ex-smoker; smoker (<10 cigarettes/d); smoker (10–19 cigarettes/d); or smoker (≥20 cigarettes/d). Menopausal status was classified as: postmenopausal; perimenopausal; premenopausal; surgical menopause; hormone replacement therapy; or oral contraceptive pill. BMI was calculated as weight (in kilograms) divided by the square of height (in metres) and categorized according to the WHO recommendations as: underweight (<18·5 kg/m2); normal weight (≥18·5 to <25·0 kg/m2); overweight (≥25·0 to <30·0 kg/m2); or obese (≥30·0 kg/m2)( 19 ). Alcohol consumption was classified according to National Health and Medical Research Council classifications as: non-drinker; low-risk drinker (≤14 drinks/week); risky drinker (15–28 drinks/week); or high-risk drinker (>28 drinks/week)( 20 ). Self-reported health was categorized as good or poor.
Statistical analysis
The 6-year incidence of type 2 diabetes from survey 3 (2001) to survey 5 (2007) was modelled using logistic regression models. The regression coefficients reflect the relationship between the incidence of diabetes and the corresponding explanatory variables adjusted for total energy intake using the residual method described by Willett and Stampfer( Reference Willett and Stampfer 21 ). Intakes of macronutrients (g/d) were categorized as quintiles, with the lowest quintile serving as the reference category. Tests for trend were performed by entering the macronutrient variables into the regression models using the quintile number. Two models were created for each macronutrient of interest. Model 1 adjusted for lifestyle and sociodemographic factors, which were treated as categorical. Model 2 adjusted simultaneously for other fat types, fibre and energy, which were treated as continuous. A P value of <0·05 was considered statistically significant and all statistical tests were two-sided. All analyses were completed with the statistical software package SAS version 9·2.
Results
During the 6 years of follow-up, 311 incident cases of type 2 diabetes were documented. Selected baseline characteristics of the study population by quintiles of total carbohydrate, protein and fat intakes are presented in Table 1. Women with a higher intake of total carbohydrate were less likely to be physically inactive and more likely to have never smoked. Women with higher intakes of total protein were less likely to live in an urban area, have a university degree or be of normal weight and more likely to be overweight or obese. Women with a higher intake of total fat were less likely to be living in an urban area, more likely to have a have a lower level of education, be physically inactive, be obese and be regular smokers. Women in the highest quintile of total carbohydrate, protein and fat consumed higher intakes of saturated fats, monounsaturated fats, fibre and energy.
Table 1 Baseline characteristics of 8370 middle-aged Australian women who completed the third survey of the ALSWH in 2001 according to quintile of dietary macronutrient intakes*
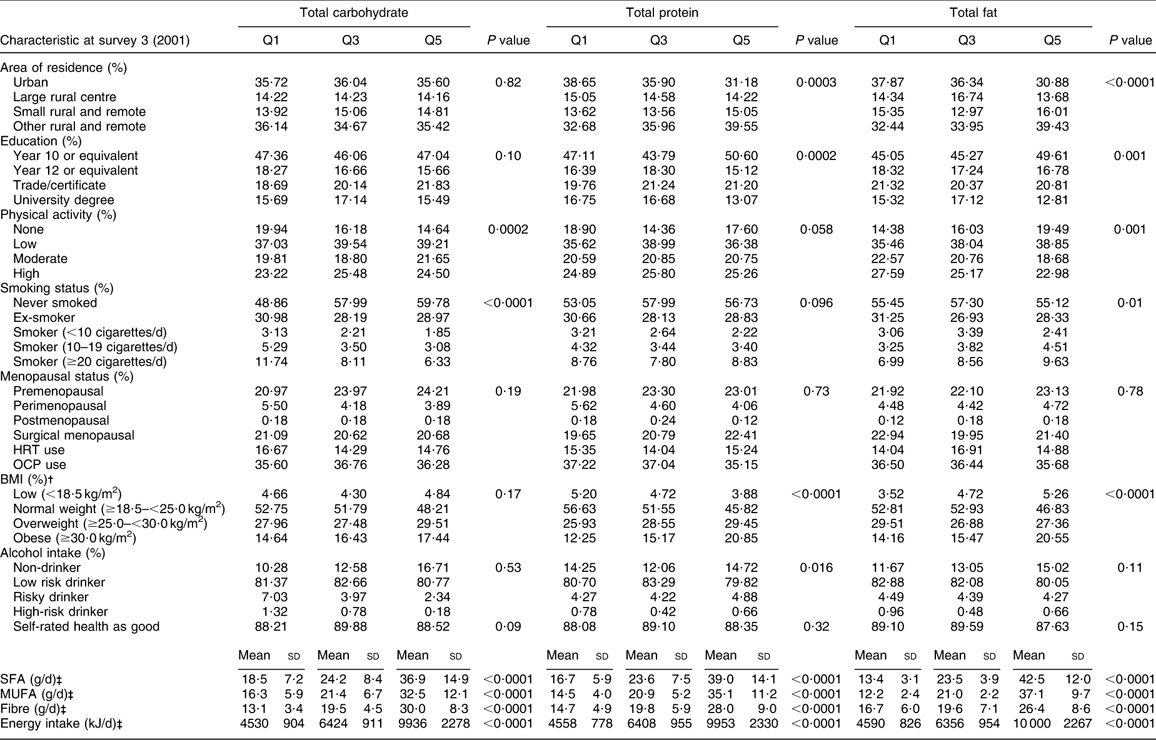
ALSWH, Australian Longitudinal Study on Women's Health; HRT, hormone replacement therapy; OCP, oral contraceptive pill.
*Data are expressed as percentage (%); P value obtained using χ 2 test of association.
†[Weight (kg)]/[height (m)]2.
‡Data are expressed as mean and standard deviation; P value obtained using ANOVA.
Relative risks (RR) of type 2 diabetes and 95 % confidence intervals for the highest quintile of total dietary macronutrient intake compared with the lowest quintile are presented in Table 2. After adjustment for potential confounding variables, no statistically significant association was observed between type 2 diabetes and intake of total carbohydrate (RR = 0·97, 95 % CI·0·61, 1·55; P = 0·63), total protein (RR = 0·88, 95 % CI 0·61, 1·27; P = 0·32) or total fat (RR = 1·27, 95 % CI 0·84, 1·91; P = 0·25).
Table 2 Relative risk of type 2 diabetes by quintile of macronutrient intakes among middle-aged Australian women from ALSWH, 2001–2007

ALSWH, Australian Longitudinal Study on Women's Health; RR, relative risk.
*Adjusted for area of residence, education, current smoking status, physical activity, self-rated health as good, menopausal status, BMI and alcohol consumption.
†As model 1 with additional adjustments for total energy intake (kJ/d) and: SFA and MUFA intakes for total carbohydrate; SFA, MUFA and fibre intakes for total protein; and fibre intake for total fat.
Relative risks and 95 % confidence intervals for type 2 diabetes by dietary fatty acid intakes are presented in Table 3. After controlling for potential confounding variables, type 2 diabetes risk was significantly associated with a higher intake of MUFA (RR = 1·64, 95 % CI 1·06, 2·54; P = 0·04), total n-3 PUFA (RR = 1·55, 95 % CI 1·03, 2·32; P = 0·01), α-linolenic acid (ALA; RR = 1·84, 95 % CI 1·25, 2·71; P < 0·01) and total n-6 PUFA (RR = 1·60, 95 % CI 1·03, 2·48; P = 0·04). There was no statistically significant association between SFA or PUFA intake and type 2 diabetes risk. In addition, neither individual intakes of marine sources of n-3 PUFA (DHA and EPA) nor the combined intake of EPA and DHA was significantly associated with type 2 diabetes risk. Although intakes of both n-3 PUFA and n-6 PUFA were positively related with type 2 diabetes risk, the ratio of n-6 PUFA to n-3 PUFA was not significantly associated with type 2 diabetes risk (RR = 1·14, 95 % CI 0·80, 1·64; P = 0·83).
Table 3 Relative risk of type 2 diabetes by quintile of fatty acid intakes among middle-aged Australian women from ALSWH, 2001–2007
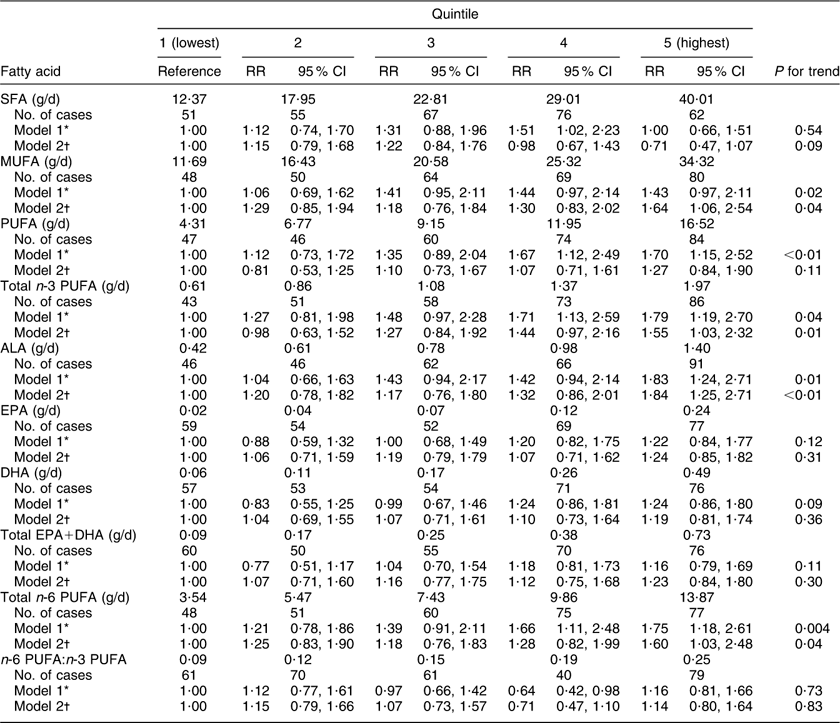
ALSWH, Australian Longitudinal Study on Women's Health; RR, relative risk; ALA, α-linolenic acid.
*Adjusted for area of residence, education, current smoker status, physical activity, self-rated health as good, menopausal status, BMI and alcohol consumption.
†As model 1 with additional adjustments for total energy intake (kJ/d), fibre and specific types of fat entered into the model simultaneously.
Discussion
The aim of the present study was to investigate the association between dietary macronutrient intake and type 2 diabetes risk in middle-aged Australian women. After adjustment for established risk factors of type 2 diabetes, high intakes of MUFA, total n-6 PUFA and total n-3 PUFA were positively associated with type 2 diabetes risk. Further examination of the source of n-3 PUFA revealed that a higher ALA intake, but not EPA or DHA, was significantly associated with type 2 diabetes risk.
Epidemiological data on the association between dietary fat and type 2 diabetes risk are inconclusive. While dietary MUFA has tended to be neutral regarding type 2 diabetes risk in some earlier studies( Reference Meyer, Kushi and Jacobs 10 , Reference Salmeron, Hu and Manson 11 , Reference van Dam, Willett and Rimm 22 – Reference Hu, van Dam and Liu 24 ), a positive association was observed with MUFA and type 2 diabetes in the current study and in some previous studies( Reference Hodge, English and O'Dea 25 , Reference Feskens, Virtanen and Rasanen 26 ). Dietary MUFA is also associated with impaired glucose tolerance( Reference Marshall, Hoag and Shetterly 27 ) and fasting serum insulin( Reference Mayer, Newman and Quesenberry 28 ). Given that MUFA intake is highly correlated with animal fat (SFA) in a typical Western dietary pattern as they are commonly found in the same foods, the current result did not change after controlling for SFA. Increased type 2 diabetes risk with total n-3 PUFA intake observed in the present study is in agreement with the results of a double-blinded controlled study which showed that a high intake of n-3 PUFA moderately increased blood glucose and decreased insulin sensitivity( Reference Mostad, Bjerve and Bjorgaas 29 ). In the Insulin Resistance Atherosclerosis study, higher intake of n-3 PUFA was associated with a lower insulin sensitivity among obese individuals( Reference Mayer-Davis, Monaco and Hoen 30 ). A cross-sectional study found a positive relationship between dietary ALA and fasting insulin level( Reference Djousse, Hunt and Tang 31 ). In the Melbourne Collaborative Cohort Study, ALA was positively associated with a higher risk of type 2 diabetes before controlling for BMI( Reference Hodge, English and O'Dea 25 ). Other investigators, however, reported contradictory results for total n-3 PUFA and ALA. No association was found between total n-3 PUFA intake and glucose metabolism( Reference Toft, Bonaa and Ingebretsen 32 ), fasting insulin( Reference Hartweg, Perera and Montori 33 ) or type 2 diabetes risk( Reference Kröger, Zietemann and Enzenbach 34 ). Two clinical trials showed no beneficial effect of ALA in the form of flaxseed oil on insulin response and glycaemic control( Reference Barre, Mizier-Barre and Griscti 35 , Reference Taylor, Noto and Stringer 36 ). Other studies suggested a protective effect of ALA against type 2 diabetes risk( Reference Brostow, Odegaard and Koh 37 – Reference Muramatsu, Yatsuya and Toyoshima 39 ). Although the underlying mechanisms responsible for the positive association between ALA intake and type 2 diabetes risk remain unclear, an in vitro study suggested that ALA stimulated the development of a pro-inflammatory environment within the vascular endothelium( Reference Toborek, Lee and Garrido 40 ), which is a marker of type 2 diabetes( Reference Williams and Nadler 41 ). Findings from an animal study indicated that feeding hamsters a diet rich in ALA for 9 weeks resulted in a 40 % decrease in insulin secretion compared with hamsters fed a diet rich in SFA( Reference Morise, Serougne and Gripois 42 ).
A substantial body of evidence highlights the importance of reducing n-6 PUFA intake in order to decrease the adverse health effects of excess arachidonic acid and its eicosanoid products. Intake of n-6 PUFA has also been linked with CVD and increased inflammatory markers( Reference Simopoulos 43 ). In the present study, n-6 PUFA was positively associated with type 2 diabetes risk. This finding is consistent with cross-sectional studies with questionnaire-assessed diet intake( Reference Mooy, Grootenhuis and de Vries 44 ). A 10-year prospective study reported that individuals who developed diabetes had higher proportions of γ-linolenic acid (18 : 3n-6) and dihomo-γ-linolenic acid (20 : 3n-6) in serum cholesterol esters at baseline than did normal individuals( Reference Vessby, Aro and Skarfors 45 ). In a nested case–cohort design, erythrocyte 18 : 3n-6 was directly related to type 2 diabetes risk( Reference Kröger, Zietemann and Enzenbach 34 ). Similarly, a nested case–referent study showed that adrenic acid (22 : 4n-6) was associated with increased diabetes risk( Reference Krachler, Norberg and Eriksson 46 ). In a large cross-sectional study of elderly Swedish men, n-6 PUFA content of adipose tissue was also negatively associated with insulin sensitivity( Reference Iggman, Arnlov and Vessby 47 ).
The present investigation did not find evidence for an effect of total carbohydrate intake on type 2 diabetes risk, consistent with results of previous cohort studies( Reference Meyer, Kushi and Jacobs 4 , Reference Colditz, Manson and Stampfer 7 , Reference Schulze, Schulz and Heidemann 48 ). The lack of an association between total protein intake and type 2 diabetes risk observed in the present study is consistent with a recent meta-analysis( Reference Alhazmi, Stojanovski and McEvoy 49 ). The lack of association between intakes of SFA and total PUFA and type 2 diabetes risk in the current study was also observed in the Nurses’ Health Study( Reference Colditz, Manson and Stampfer 7 , Reference Salmeron, Hu and Manson 11 ) and the Iowa Women's Health Study( Reference Meyer, Kushi and Jacobs 10 ). Similarly, dietary EPA and DHA were not significantly associated with type 2 diabetes risk in the current study, supporting findings of two meta-analyses based on randomized controlled trails( Reference Akinkuolie, Ngwa and Meigs 50 , Reference Montori, Farmer and Wollan 51 ). Previous evidence showed that n-3 PUFA and n-6 PUFA metabolize to longer-chain fatty acids and that an excessive amount of one may imbalance the protective role of the other( Reference Deckelbaum and Torrejon 52 ). However, in the present study there was no evidence that n-6 PUFA:n-3 PUFA is associated with type 2 diabetes risk, supporting findings from the Singapore Chinese Health Study( Reference Brostow, Odegaard and Koh 37 ).
The major strengths of the current study include the large representative sample of Australian women of similar age, the availability of detailed information on important lifestyle and sociodemographic factors, and the use of an FFQ that was specifically designed and validated in the Australian population. Limitations of the current analysis should also be noted. The possibility that the observed positive association between fatty acids and type 2 diabetes was due to bias should be considered. Furthermore, residual confounding cannot be fully omitted as a potential explanation for the current observations. It is conceivable that there are other confounders that were not controlled for or were unable to be completely controlled for and this may potentially have led to an overestimate of the association. Because screening for blood glucose level was not feasible in this large cohort, misclassification of participants with undetected diabetes is likely due to the self-reporting. However, the validation study of self-reported diabetes revealed that diabetes reporting was confirmed in 70 % of self-reported diabetics in this population( Reference Lowe, Byles and Dolja-Gore 16 ). This is similar to results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study, in which 72 % of the self-reported cases documented by mailed questionnaires were confirmed( Reference Sluijs, Beulens and van der A 6 ); and compares favourably with the findings from the Iowa Women's Health Study in which diabetes was confirmed in 64 % of self-reported cases( Reference Meyer, Kushi and Jacobs 4 ).
Non-differential misclassification of dietary exposure is likely which may potentially dilute the effect estimates found, contributing to the lack of association observed between some macronutrients and type 2 diabetes risk. In addition, a baseline dietary assessment limited the ability to account for possible changes over time in macronutrient intake. However, due to the relatively short period of follow-up (6 years), the dietary patterns of women are unlikely to have changed considerably.
Conclusion
The present data suggest that MUFA, total n-3 PUFA and total n-6 PUFA intakes are associated with increased type 2 diabetes risk in women. These relationships require further investigation using an objective method. The possible biological mechanisms behind the observed association need further investigation.
Acknowledgements
Sources of funding: The ALSWH, which is conducted by researchers at the University of Newcastle and the University of Queensland, is funded by the Australian Government Department of Health and Ageing. The Australian Government Department of Health and Ageing had no role in the in the design, analysis or writing of this article. Conflicts of interest: The authors declare that they have no conflicts of interest. Authors’ contributions: All authors were involved in the conception and design of the study. All authors critically reviewed the manuscript and approved the final version for publication. Acknowledgements: The authors thank all participants for their valuable contribution to this project. They are also grateful to Professor Wendy Brown, University of Queensland, for her critical review of this manuscript.





