Introduction
The black truffle, Tuber melanosporum Vittad. (Pezizales: Tuberaceae), is a hypogeal fungus that establishes a mycorrhiza relationship mainly with the Quercus genus (Bonito et al. Reference Bonito, Gryganskyi, Trappe and Vilgalys2010) that has been traditionally collected from wild forests and in the last decades cultivated due to its high gastronomic value and economic interest (Oliach et al. Reference Oliach, Morte, Sánchez, Navarro-Ródenas, Marco, Gutiérrez, Martín-Santafé, Fischer, Albisu, García-Barreda, Colina, Sánchez-González, Calama and Bonet2020). T. melanosporum usually starts to fructify when the host tree is about 6–7 years old and continues until it is 20–25 years old, when the production starts to drop (Martín-Santafé, Reference Martín-Santafé2020). During this period, the mycorrhiza develops a burnt area around the host tree where the herbaceous cover is scarce (Splivallo et al. Reference Splivallo, Ottonello, Mello and Karlovsky2011) due to the production of mainly two truffle volatiles (ethylene and indole-3-acetic acid) that act as potent herbicides at high concentrations (Hansen and Grossmann Reference Hansen and Grossmann2000; Grossmann Reference Grossmann2003). Moreover, alkaline soils are required for its development, with pH that ranges from 7.0 to 8.9, with a median of 7.9 (Jaillard et al. Reference Jaillard, Oliach, Sourzat, Colinas, Zambonelli, Iotti and Murat2016). This fungus is also benefited by high organic matter content and balanced textures, generating loamy soils that are well-structured, porous, aerated and without excess of water (Jaillard et al. Reference Jaillard, Oliach, Sourzat, Colinas, Zambonelli, Iotti and Murat2016).
The economic importance of this fungus has led to a monoculture situation that along with certain agricultural practices like irrigation have favoured the presence of some insect species that then become pests (Martín-Santafé Reference Martín-Santafé2020). The European truffle beetle, Leiodes cinnamomeus (Panzer) (Coleoptera: Leiodidae), is one of the most serious pests in black truffle plantations (Arzone Reference Arzone1971; Martín-Santafé et al. Reference Martín-Santafé, Pérez-Fortea, Zuriaga and Barriuso-Vargas2014; Navarro-Llopis et al. Reference Navarro-Llopis, López, Primo, Martín-Santafé and Vacas2021). Adults and larvae feed on T. melanosporum fruiting bodies, causing galleries which reduce quality and can generate up to 70% of economic losses in plantations (Barriuso et al. 2012). Cultural practices, such as frequent collections of truffles and the use of traps for mass capture of adults, are recommended (Martín-Santafé et al. Reference Martín-Santafé, Pérez-Fortea, Zuriaga and Barriuso-Vargas2014; Navarro-Llopis et al. Reference Navarro-Llopis, López, Primo, Martín-Santafé and Vacas2021). However, these practices are not enough to reduce the population of L. cinnamomeus to acceptable levels. Thus, alternative biological control methods are needed.
Entomopathogenic nematodes (EPNs) are a group of species that have been studied and used as biological control agents for decades (Lacey and Georgis Reference Lacey and Georgis2012; Shapiro-Illan et al. Reference Shapiro-Ilan, Hazir, Glazer and Lacey2017). These nematodes, species of the genus Steinernema and Heterorhabditis, which are the most common, are widely distributed throughout the world and have been reported from a wide variety of soils (Hominick Reference Hominick and Gaugler2002). The presence and survival of EPNs are influenced by multiple factors, including geographical location, climatic conditions, habitat type and soil properties, such as pH, organic matter content and texture (Stuart et al. Reference Stuart, Barbercheck, Grewal and Campos-Herrera2015). EPNs are believed to be adapted to the soil-specific conditions where they were isolated (Kung et al. Reference Kung, Gaugler and Kaya1991). Julià et al. (Reference Julià, Morton and Garcia-del-Pino2023) have already observed the susceptibility of L. cinnamomeus adults and larvae to EPNs under laboratory conditions, suggesting that the presence of these nematodes in truffle plantations could naturally regulate the population of this beetle.
Free-living bacterivorous nematodes (FLBNs) species can interfere and compete with EPNs for host resources (Duncan et al. Reference Duncan, Dunn, Bague and Nguyen2003; Campos-Herrera et al. Reference Campos-Herrera, El-Borai and Duncan2012). Some species of the genus Pristionchus exhibit facultative insect parasitic, necromenic and nematophagous behaviour (Félix et al. Reference Félix, Ailion, Hsu, Richaud and Wang2018). For example, Pristionchus pacificus Sommer, Carta, Kim and Sternberg (Rhabditida: Diplogastridae) can display dimorphic mouth structures, differing in the number and shape of teeth and in the complexity of other mouth armature. This dimorphism enables it to adopt a predator behaviour towards other nematodes when bacterial food is scarce (Meyer et al. Reference Meyer, Baskaran, Quast, Susoy, Rodelsperger, Glockner and Sommer2017). Moreover, previous studies have also observed that species such as P. pacificus and Pristionchus maupasi (Potts) (Rhabditida: Diplogastridae) form necromenic or phoretic associations with various species of beetles (Herrmann et al. Reference Hermann, Mayer and Sommer2006, Hong et al. Reference Hong, Svatos, Herrmann and Sommer2008; Félix et al. Reference Félix, Ailion, Hsu, Richaud and Wang2018).
The discovery of new strains and species of EPNs has been important in their commercial success as biocontrol agents against pests (Shapiro-Ilan et al. Reference Shapiro-Ilan, Gouge, Koppenhofer and Gaugler2002; Lacey and Georgis Reference Lacey and Georgis2012) due to the importance of being adapted to the environmental conditions of the site of application (Bedding Reference Bedding1990). There is currently a lack of studies examining the presence of EPNs in truffle soils. Therefore, the main objectives of this research were: (1) to isolate EPNs and study their ecological requirements in truffle soils from the regions of Teruel and Catalonia (Spain); (2) assess the presence of species of Pristionchus nematodes that could interfere with the presence of EPNs.
Material and methods
Field sampling and soil characterization
A total of 164 soil samples in 112 and 52 locations of Teruel and Catalonia (Spain), respectively, were collected from different black truffle-growing areas (Figure 1) from autumn (October 2020) to spring (March 2021). Each soil sample weighed about 1 kg, resulted from the mixture of four subsamples of about 200 cm3 dug from 0 to 20 cm deep in soil around the host tree (Campbell et al. Reference Orza, Yoder, Lewis and Gaugler1998).

Figure 1. Geographical distribution of EPN sampling locations in the regions of A. Teruel and B. Catalonia (Spain). Green triangles: sites with S. feltiae. Red triangles: sites with H. bacteriophora. White circles: sites without nematodes.
In 21 productive plantations in Teruel, both nonburnt areas (without the presence of T. melanosporum) and burnt areas were sampled to assess whether the volatiles of T. melanosporum, which act as herbicides, influenced the occurrence of nematodes. The sampling methodology employed was the same as explained above.
The locations and altitudes of the sampled soils were recorded using global positioning system equipment. Data of annual average air temperature and rainfall were recorded from maps of the Government of Aragon and Government of Catalonia. The study area in Teruel lies between 888 and 1760 m above sea level, with mean annual temperatures of 9–13 ºC and mean annual rainfall of 400–600 mm. In the case of Catalonia, the study area lies between 514 and 1484 m above sea level, with mean annual temperatures of 8–12 ºC and mean annual rainfall of 600–800 mm. The samples were categorized based on habitat type, with 20.1% obtained from forests known to naturally produce T. melanosporum, 67.1% from productive truffle plantations and 12.8% from low-productive or nonproductive plantations. This last category includes plantations less than 6 years old or more than 25 years old.
For each collected sample, pH, soil organic content and soil particle size were measured. The soil pH was measured from a 1:2.5 soil/mQ-water suspension and the total organic matter was determined by wet oxidation (MAPA 1975). To determine the soil texture, particle size analysis was performed to calculate the percentage of silt, sand and clay using the Bouyoucos method (MAPA 1975).
Isolation of nematodes
Isolation of EPNs
The insect bait method (Galleria trap) described by Bedding and Akhurst (Reference Bedding and Akhurst1975) was used to isolate EPNs. For each soil sample, five Petri dishes containing five last-stage larvae of Galleria mellonella (Linnaeus) (Lepidoptera: Pyralidae) were covered with soil. After incubating for one week at 24 ºC, dead insects that showed symptoms of infection were rinsed in sterile Ringer solution and placed individually into modified White traps (White 1927) to collect the emerged infective juveniles (IJs). The assay was conducted twice. To confirm their pathogenicity, harvested nematode strains were reared at 24 °C in last instar larvae of G. mellonella, according to the method of Woodring and Kaya (Reference Woodring and Kaya1988). They were maintained at 9 °C until their molecular identification.
Isolation of Pristionchus nematodes
The presence of Pristionchus and other FLBNs was determined using a similar methodology. For each soil sample, one Petri dish containing three frozen dead last-stage larvae of G. mellonella was covered with soil. After incubating for one week at 24 ºC, nematodes detected around the G. mellonella cadavers were collected in Eppendorf tubes with ethanol 70%. The assay was conducted twice.
The presence of Pristionchus nematodes was also determined directly from the surface of black truffle fruit bodies to confirm their presence on this fungus. Eight fruit bodies of T. melanosporum were sampled in Teruel. The nematodes around them were rinsed and collected in Eppendorfs with ethanol 100%. Their presence was also determined under the elytra of L. cinnamomeus to confirm the phoretic relationship between nematode and insect. Twenty Petri dishes with nutrient agar were used, with two elytra per dish. After 7–10 days of incubation, the percentage of Petri dishes with Pristionchus nematodes was calculated.
Identification of nematodes
EPNs and Pristionchus nematodes isolated were identified using molecular techniques. For each sample, a PCR reaction was performed to amplify an internal transcribed spacer (ITS) region from genomic DNA extracted from a single female. PCR amplification conditions followed procedures described by Hominick et al. (Reference Hominick, Briscoe, del Pino, Heng, Hunt, Kozodoy, Macrek, Nguyen, Reid, Spiridonov, Stock, Sturhan, Waturu and Yoshida1997) using the primers 18s (5-AAAGATTAAGCCATGCATG-3) and 26s (5-CATTCTTGGCAAATGCTTTCG-3) (Vrain et al. Reference Vrain, Wakarchuk, Levesque and Hamilton1992). Amplified samples were purified and sequenced before being compared with GenBank database sequences of Steinernema, Heterorhabditis and Pristionchus using Blastn (NCBI; http://www.ncbi.nlm.nih.gov), searching for sequence similarity matches at ≥ 98%.
An alignment of the ITS rDNA sequences was generated using Clustal W (Thompson et al. Reference Thompson, Gibson, Plewniak, Jeanmougin and Higgins1997) for Steinernema and Heterorhabditis species separately. Phylogenetic analyses were performed using the maximum parsimony (MP) method with MEGA X software (Kumar et al. Reference Kumar, Stecher, Li, Knyaz and Tamura2018). The calculated phylogenetic trees were evaluated by bootstrap analysis based on 1000 replicates. Caenorhabditis elegans (Maupas) (X03680) was used as outgroup during calculation of the trees based on ITS sequences.
Data analysis
The data for EPNs and Pristionchus were assessed in relation to environmental and soil factors, using the recovery frequency (number positive samples/number total samples), and the number and percentage of positive samples per variable category. The data corresponding with EPNs and Pristionchus were analysed separately. Generalized linear model (GLM), with negative binomial distribution and a log link function, was used to examine the relationships between the nematodes and the environmental/soil variables. The variables were categorized into groups, as shown in Tables 1 and 2. The recovery frequency of nematodes obtained in the regions of Catalonia and Teruel and in the burnt and nonburnt areas were also compared using GLM with negative binomial distribution. All data were analysed with the R software (version 4.2.2) (R Core Team 2022). Any comparison was considered significant if the p-value was less than 0.05.
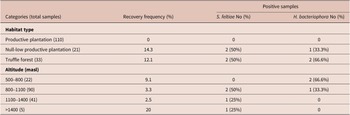
Table 1. Distribution of EPNs at different environmental variables

Table 2. Distribution of EPNs at different physical–chemical variables

Results
Isolation of entomopathogenic nematode species
Entomopathogenic nematodes were isolated from 7 of 164 soil samples (4.3%) distributed across plantations and wild truffle-producing forests. EPNs were recovered from 3 of 112 samples from Teruel (2.6%) and 4 of 52 samples from Catalonia (7.7%), with no significant differences between both regions (χ2 = 1.92, df = 1, p = 0.17).
Morphologic and molecular examinations revealed the presence of four isolates of Steinernema and three isolates of Heterorhabditis. BLASTn analysis of the ITS region showed that the three steinernematid recovered from Teruel and one from Catalonia shared sequence similarity of >99% with Steinernema feltiae (Filipev) (Panagrolaimida: Steinernematidea) (Figure 2). The second species isolated from three samples in Catalonia shared sequence similarity of >99% with Heterorhabditis bacteriophora (Poinar) (Rhabditida: Heterorhabditidae) (Figure 3).

Figure 2. Phylogenetic relationship of isolated S. feltiae strains from Teruel (TE15, TE104 and TE112) and Catalonia (CT3) and published ITS sequences of Steinernema species using maximum parsimony tree. C. elegans was used as the outgroup. Numbers before species names correspond to GenBank accession numbers.

Figure 3. Phylogenetic relationship of isolated H. bacteriophora strains from Catalonia (CT2, CT47 and CT52) and published ITS-sequences of Heterorhabditis species using maximum parsimony tree. C. elegans was used as the outgroup. Numbers before species names correspond to GenBank accession numbers.
EPN distribution in relation to environmental and soil characteristics
The habitat type significantly affected the recovery frequency of EPNs (χ2 = 16.25, df = 2, p < 0.05). Both species were isolated in truffle forest (12.1% of this habitat) and null-low productive plantations (14.3% of this habitat), but no EPNs were detected in productive plantations (Table 1). Altitude did not affect the occurrence of EPNs (χ2 = 2.99, df = 3, p = 0.39). However, H. bacteriophora strains were isolated at lower altitudes (694, 767 and 804 masl) than S. feltiae (899, 942, 1336 and 1417 masl) (χ2 = 6.41, df = 1, p < 0.05).
EPN distribution was also examined according to soil pH, organic matter and texture. Both EPN species isolated were found in alkaline soil samples with pH ranging from 7.58 to 8.47 (Table 2). However, there was no significant effect of soil pH to EPNs occurrence (χ2 = 4.83, df = 3, p = 0.18). Instead, the recovery frequency of S. feltiae and H. bacteriophora was significantly influenced by the content of organic matter (χ2 = 12.85, df = 5, p < 0.05), as these nematodes were predominantly isolated from soils with higher organic matter content (2.1–11.04%). The occurrence of EPNs was not significantly influenced by soil texture (χ2 = 9.06, df = 6, p = 0.17), although they were detected in sandy loam (16.7%), loam (3.4%) and sandy clay loam (2.7%) soils but not in silt loam, clay loam, sandy clay and clay soils (Table 2).
Presence of Pristionchus species
Species of genus Pristionchus were detected in 46 of 164 soil samples (28%) distributed across plantations and truffle forests surveyed. Individuals were recovered from 35 of 112 samples in Teruel (31.3%) and 11 of 52 samples in Catalonia (21.2%), with no significant differences between both regions (χ2 = 1.85, df = 1, p = 0.17).
BLASTn analysis of the ITS region showed that most of these isolates (39 of 46) shared sequence similarity of >98% with P. maupasi. The other isolates (7 of 46) were not similar to published ITS sequences of any specific species of this genus. Hence, they were considered as Pristionchus sp. Other free-living bacteriophage nematode species were also recovered and identified from nine samples in Teruel (8%) and six samples in Catalonia (11.5%) (Table 3).
Table 3. Number of soil samples with other identified species of free-living bacteriophage nematodes

BLASTn analysis also identified P. maupasi species around the eight fruit bodies of T. melanosporum sampled, sharing sequence similarities of >99% with this species. Moreover, Pristionchus nematodes were identified under the elytra of 45% of L. cinnamomeus adults. Some other nonidentified nematodes were also detected.
Pristionchus distribution in relation to environmental and soil characteristics
Pristionchus nematodes were isolated in all three sampled habitat types (χ2 = 1.15, df = 2, p = 0.56), including productive plantations (28.2%), null-low productive plantations (33.3%) and truffle forests (21.2%) (Table 4). Furthermore, these nematodes were found across all altitudes sampled (χ2 = 1.77, df = 3, p = 0.62).
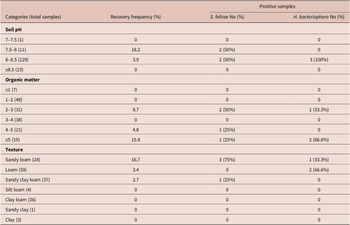
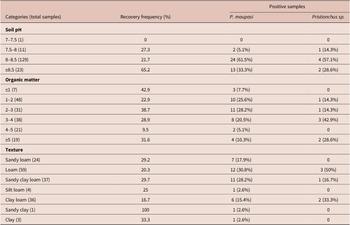
Table 4. Distribution of Pristionchus at different environmental variables

Pristionchus distribution was also examined according to soil pH, organic matter and texture. None of these variables significantly influenced the presence of Pristionchus (χ2 = 3.55, df = 3, p = 0.31; χ2 = 6.47, df = 5, p = 0.26 and χ2 = 4.1, df = 6, p = 0.66, respectively). All nematodes were found in alkaline soil samples (7.75 to 8.7 pH), in all seven types of texture sampled with variable organic matter content (0.73–5.92%) (Table 5).
Table 5. Distribution of Pristionchus at different physical–chemical variables

Presence of EPNs and Pristionchus in burnt and nonburnt areas
The number of isolated EPNs was not statistically analysed because no nematodes were recovered from the 42 samples (21 from burnt areas and 21 from nonburnt areas). In the case of Pristionchus, its recovery frequency was found to be significantly higher in burnt areas (38.1%) compared to nonburnt areas (4.8%) (χ2 = 7.69, df = 1, p < 0.05) (Table 6).
Table 6. Distribution of Pristionchus at burnt and nonburnt areas

Discussion
This study represents the first survey aimed to isolate native EPN species and necromenic Pristionchus nematodes in truffle soils. Despite a relatively low prevalence of EPNs (7 of 164 samples; 4.3%), our findings are consistent with previous studies. Globally, EPN prevalence in soil samples has been reported to range from 0.7% to 50% (Hominick Reference Hominick and Gaugler2002). In Spain, Morton and Garcia del Pino (Reference Morton and García-del-Pino2009) observed an incidence of 5.2% in stone fruit orchards in Catalonia, which aligns with the results of our study. Similar results were obtained by Campos-Herrera et al. (Reference Campos-Herrera, Escuer, Labrador, Robertson, Barrios and Gutiérrez2007) in soils of La Rioja (5.4%). In contrast, Garcia del Pino and Palomo (Reference Garcia del Pino and Palomo1996) recovered EPNs in 28.2% of cultivated soils and in 16.3% of woodlands in Catalonia.
In our study, the diversity of EPNs was found to be low, with only two species isolated: S. feltiae and H. bacteriophora. These findings are consistent with previous studies conducted in Mediterranean countries, which have also reported low diversity of EPNs (Garcia del Pino and Palomo Reference Garcia del Pino and Palomo1996; Campos-Herrera et al. Reference Campos-Herrera, Escuer, Labrador, Robertson, Barrios and Gutiérrez2007; Morton and Garcia del Pino Reference Morton and García-del-Pino2009; Noujeim et al. Reference Noujeim, Khater, Pages, Ogier, Tailliez, Hamze and Thaler2011; Valadas et al. Reference Valadas, Laranjo, Mota and Oliveira2013; Tarasco et al. Reference Tarasco, Triggiani, Sai and Zamoum2009, Reference Tarasco, Clausi, Rappazzo, Panzavolta, Curto, Sorino, Oreste, Longo, Leone, Tiberi, Vinciguerra and Triggiani2015; Benseddik et al. Reference Benseddik, Boutaleb Joutei, Blenzar, Ezrari, Molina, Radouane, Mokrini, Tahiri, Lahlali and Dababat2020). Four new strains of S. feltiae were isolated, being the most common species detected globally (57%) and in the region of Teruel (100%). In fact, S. feltiae is frequently reported as the dominant EPN species in Mediterranean countries, accounting for 38% in Italy (Tarasco et al. Reference Tarasco, Clausi, Rappazzo, Panzavolta, Curto, Sorino, Oreste, Longo, Leone, Tiberi, Vinciguerra and Triggiani2015), 55% (Campos-Herrera et al. Reference Campos-Herrera, Escuer, Labrador, Robertson, Barrios and Gutiérrez2007) and 70% in Spain (Garcia del Pino and Palomo, Reference Garcia del Pino and Palomo1996), 75% in Portugal (Valadas et al. Reference Valadas, Laranjo, Mota and Oliveira2013), 71–85% in Turkey (Hazir et al. Reference Hazir, Keskin, Stock, Kaya and Ozcan2003; Yuksel and Canhilal Reference Yuksel and Canhilal2019; Gümüş Askar et al. Reference Gümüş Askar, Yüksel, Öcal, Özer, Kütük, Dababat and İmren2022) and 87% in Algeria (Tarasco et al. Reference Tarasco, Triggiani, Sai and Zamoum2009). In the region of Catalonia, three strains of H. bacteriophora were identified and were the most common species detected (75%). This species was isolated in inland areas at lower altitudes (694–807 masl) compared to S. feltiae (899–1417 masl). Most studies have reported that H. bacteriophora is commonly found in maritime environments, where it tends to be more prevalent than steinernematids (Rosa et al. Reference Rosa, Bonifassi, Amaral, Lacey, Simoes and Laumond2000; Emelianoff et al. Reference Emelianoff, Le Brun, Pagès, Stock, Tailliez, Moulia and Sicard2008). However, our results align with other reports, suggesting that H. bacteriophora can also be the dominant species in inland areas (Benseddik et al. Reference Benseddik, Boutaleb Joutei, Blenzar, Ezrari, Molina, Radouane, Mokrini, Tahiri, Lahlali and Dababat2020).
The measured prevalence of EPN is influenced by various factors, including sampling method, insect bait and environmental and soil characteristics such as habitat type, altitude, soil texture, moisture level, organic matter, pH and biotic factors (Garcia del Pino and Palomo Reference Garcia del Pino and Palomo1996; Benseddik et al. Reference Benseddik, Boutaleb Joutei, Blenzar, Ezrari, Molina, Radouane, Mokrini, Tahiri, Lahlali and Dababat2020). In our study, we found that habitat type significantly influenced the recovery frequency of EPNs. Although productive plantations are subjected to more frequent irrigation compared to null-low productive plantations and truffle forests, EPNs were only isolated from the latter two habitats, and none were isolated from productive plantations. T. melanosporum is associated with the development of burnt area around the host tree where the herbaceous cover is scarce (Splivallo et al. Reference Splivallo, Ottonello, Mello and Karlovsky2011). This characteristic is more prominent in productive plantations, potentially leading to a loss of diversity among potential insect hosts (Campos-Herrera et al. Reference Campos-Herrera, Escuer, Labrador, Robertson, Barrios and Gutiérrez2007). Although highly intensive monoculture areas can produce outbreaks of insect pest species susceptible to EPNs infection (Campos-Herrera et al. Reference Campos-Herrera, Escuer, Labrador, Robertson, Barrios and Gutiérrez2007), the beetle L. cinnamomeus develops during the coldest period of the year when low temperatures are suboptimal for EPN infection (Julià et al. Reference Julià, Morton and Garcia-del-Pino2023). Moreover, the high recovery rates of the genus Pristionchus observed in burnt areas could indicate that the herbicides emitted by T. melanosporum may not have a nematicidal effect on nematodes, including EPNs. Thus, the greater presence of vegetation in null-low productive plantations and wild truffles could positively influence the presence of EPNs by providing increased diversity of potential insect hosts during spring and summer.
In our survey, EPNs were recovered from soils with moderate to high sand content. Previous studies observed that EPNs were commonly found in soils with high sand content (Stock et al. Reference Stock, Pryor and Kaya1999; Valadas et al. Reference Valadas, Laranjo, Mota and Oliveira2013; Tarasco et al. Reference Tarasco, Clausi, Rappazzo, Panzavolta, Curto, Sorino, Oreste, Longo, Leone, Tiberi, Vinciguerra and Triggiani2015), suggesting that light textured soils improve the mobility and survival of EPNs (Stock et al. Reference Stock, Pryor and Kaya1999) compared to heavy textured soils, which clay content could difficult the EPNs movement and affect their recovery (Mráček et al. Reference Mráček, Bečvář, Kindlmann and Jersáková2005). Furthermore, organic matter content has also been observed to positively correlate with the occurrence of EPNs (Hominick and Briscoe Reference Hominick and Briscoe1990; Alumai et al. Reference Alumai, Grewal, Hoy and Willoughby2006; Canhilal and Carner Reference Canhilal and Carner2006). Our results agree with these studies, in which both EPN species were significantly more prevalent in soils with higher organic matter. Additionally, all strains of S. feltiae and H. bacteriphora were isolated from alkaline soils because T. melanosporum requires soils with high pH levels, ranging from 7.0 to 8.9 (Jaillard et al. Reference Jaillard, Oliach, Sourzat, Colinas, Zambonelli, Iotti and Murat2016). Our results agree with Campos-Herrera et al. (Reference Campos-Herrera, Escuer, Labrador, Robertson, Barrios and Gutiérrez2007), who also isolated EPNs from alkaline soils, while Khatri-Chhetri et al. (Reference Khatri-Chhetri, Waeyenberge, Manandhar and Moens2010) detected most of EPNs in acidic soils, particularly steinernermatids. Kung et al. (Reference Kung, Gaugler and Kaya1990) reported that pH values within the range of 4–8 did not significantly affect the survival and pathogenicity of EPNs, suggesting that the pH tolerance of indigenous nematodes may vary depending on the region of isolation (Khathwayo et al. Reference Khathwayo, Ramakuwela, Hatting, Shapiro-Ilan and Cochrane2021). In fact, EPNs are believed to be adapted to the specific soil conditions of the region they were isolated (Kung et al. Reference Kung, Gaugler and Kaya1991).
Biotic factors, such as FLBNs species, may also affect the presence of EPNs (Stuart et al. Reference Stuart, Barbercheck, Grewal and Campos-Herrera2015). Previous studies observed that these nematodes are able to interfere and compete with EPNs species for host resources. Laboratory experiments observed that Pellioditis sp. can compete with and even displace EPNs species that had previously killed the insect host (Duncan et al. Reference Duncan, Dunn, Bague and Nguyen2003). Another study demonstrated the ability of Acrobeloides maximum and Rhabditis rainaispecies to interfere with the development of various species of EPNs inside weevil Diaprepes abbreviatus hosts, reducing the production of new progeny (Campos-Herrera et al. Reference Campos-Herrera, El-Borai and Duncan2012). The genus Pristionchus sp. is known to be associated with different substrates, such as soil, humus, compost, moss, around roots of several species, rotten wood, stems and fruits, and decomposing fungi (Sudhaus and Fürst von Lieven Reference Sudhaus and Fürst von Lieven2003; Félix et al. Reference Félix, Ailion, Hsu, Richaud and Wang2018). In our study, Pristionchus nematodes, particularly P. maupasi, were frequently found in truffle soils (28% of all samples) and on the fruit bodies of these truffles (100%). These results agree with Kilian et al. (Reference Kilian, Kadej, Cooter and Harvey2022), who also observed the presence of unidentified nematodes around the fruit bodies of T. melanosporum. Additionally, we detected the presence of this nematode under the elytra of L. cinnamomeus (45%), which is the first report that has confirmed the necromenic/phoretic relationship between P. maupasi and L. cinnamomeus. Previous studies reported that some Pristionchus species can prey on other nematodes due to the mouth dimorphism developed by these species (Serobyan et al. Reference Serobyan, Ragsdale and Sommer2014; Wilecki et al. Reference Wilecki, Lightfoot, Susoy and Sommer2015). Moreover, we also observed predatory behaviour in P. maupasi towards EPNs under laboratory conditions (unpublished data). The co-occurrence of several other FLBN species with P. maupasi in truffle soil could indicate that these nematodes may serve as a food source in addition to bacteria. Therefore, the presence of P. maupasi may be a factor that has contributed to the low prevalence of EPNs in truffle soils.
In our study, P. maupasi was not significantly affected by environmental and soil variables. Félix et al. (Reference Félix, Ailion, Hsu, Richaud and Wang2018) observed that both dauer and feeding stages of various Pristionchus species were frequently and abundantly present on different rotting vegetal matter and decomposing fungi. They also highlight the need for more detailed studies to confirm whether the beetles that have been associated with Pristionchus spp. visit places with rotting vegetal matter, considering the seasonality of the beetle’s life cycle. In our study, L. cinnamomeus is a beetle that develops during the coldest period of the year, as it is adapted to the life cycle of T. melanosporum (Martin-Santafé et al. Reference Martín-Santafé, Pérez-Fortea, Zuriaga and Barriuso-Vargas2014). Despite the low temperatures, P. maupasi nematodes were not only isolated from truffle soils but also from T. melanosporum fruit bodies and the elytra of adult L. cinnamomeus beetles during this period. Adults of L. cinnamomeus frequently move between truffles to reproduce (Martin-Santafé 2020), visiting the fruit bodies where Pristionchus populations are present. Therefore, our results confirm the phoretic/necromenic relationship between these organisms. This is supported by the higher presence of Pristionchus in burnt areas (38.1%) than in nonburnt areas (4.8%). These results are consistent with previous studies that have observed scarab beetle species associated with Pristionchus feeding as adults on mature and rotting vegetal matter, such as Geotrupes stercorosus and Exomala orientalis (Cambefort Reference Cambefort, Hanski and Cambefort1991; Choo et al. Reference Choo, Lee, Park, Kaya, Smitley, Lee and Choo2002; Herrmann et al. Reference Hermann, Mayer and Sommer2006).
To sum up, this study has revealed the natural occurrence of S. feltiae and H. bacteriophora in truffle soils but at relatively low frequency, suggesting these EPNs might have specific adaptations to local conditions, which make them potential candidates for the development of novel biological pest control agents. However, their absence in productive plantations, where the herbaceous cover around the host tree is reduced due to T. melanosporum, might impact EPNs by limiting the diversity of available insect hosts. The high recovery frequency of P. maupasi indicates that this nematode is closely associated with truffles, having a phoretic/necromenic relationship with the beetle L. cinnamomeus. Moreover, its presence may affect the occurrence of natural populations of other nematodes, including EPNs, through competition and predation. It is possible that the low presence of EPNs in truffle soils hinders their ability to naturally regulate the population of L. cinnamomeus. However, the survival of EPNs in truffle soils during the application of inundative biological control should not pose a problem. Therefore, the potential of isolated EPNs as control agents needs to be assessed through field assays against L. cinnamomeus.
Acknowledgements
We would like to thank truffle producters of la Asociación de Truficultores de Teruel (ATRUTER) and l’Associació de Productors de Tòfona de Catalunya (PROTOCAT) for letting us survey their truffle plantations. We also wish to thank Victor Pérez Fortea for helping us during the soil sampling.
Financial support
This work was supported by Proyecto UAB/ATRUTER 202/2021 Asociación de Truficultores de Teruel (ATRUTER): Aislamiento e identificación de nematodos entomopatógenos en fincas truferas de Teruel and by Proyecto I+D+I CIBR – FITE 2021 (CITA) Gobierno de Aragón: Nuevas estrategias de control del escarabajo de la trufa en Teruel.