Introduction
Weeds decrease the aesthetic value and function of many turfgrass systems including residential and commercial lawns, golf courses, sod farms, athletic fields, and roadsides among many other sites. Weeds adversely affect surface uniformity and wear tolerance, as well as competing with desirable species for nutrients, light, and moisture, thereby reducing turfgrass health and vigor (McElroy and Martins Reference McElroy and Martins2013; Watschke et al. Reference Watschke, Dernoeden and Shetlar2013). Preemergence herbicides are commonly applied to turfgrass to control susceptible species, and some important factors that dictate the efficacy of preemergence herbicides include geography and related climatic conditions, application timing and rate, site of herbicide placement, formulation, and soil properties such as texture, organic matter content, moisture, and temperature (Knake et al. Reference Knake, Appleby and Furtick1967; McCarty and Murphy Reference McCarty, Murphy and Turgeon1994). The duration of weed control is contingent on the weed species sensitivity, herbicide persistence as influenced by physicochemical properties, and edaphic and environmental conditions, among other factors. Some of the more commonly researched annual grass and broadleaf weeds that are controlled by preemergence herbicides include annual bluegrass (Poa annua L.), crabgrass species (Digitaria spp.), goosegrass [Eleusine indica (L.) Gaertn.], dandelion (Taraxacum officinale Weber), and pigweed species (Amaranthus spp.), whereas other species have not been fully evaluated (Leon et al. Reference Leon, Unruh and Brecke2016; McElroy et al. Reference McElroy, Head, Wehtje and Spak2017; Unruh and Brecke Reference Unruh and Brecke2007). Of these herbicides, indaziflam and oxadiazon are of specific interest because of their potential efficacy in consistently controlling annual weeds throughout the emergence period (Brecke et al. Reference Brecke, Stephenson and Unruh2010; Perry et al. Reference Perry, McElroy, Doroh and Walker2011).
Indaziflam is a preemergence herbicide with moderate persistence [half-life (T 1/2) ≥ 150 d] used in turfgrass systems that provides effective long-term control of many weed species in warm-season turfgrass (Guerra et al. Reference Guerra, Oliveira Neto, Oliveira, Constantin and Takano2014; Jhala et al. Reference Jhala, Ramirez and Singh2013; Shaner Reference Shaner2014a). Indaziflam is preferred because of its low use rate of 16 to 55 g ai ha−1 compared to other preemergence herbicides, which tend to be applied at higher rates of 500 to 3,000 g ha−1 (McElroy and Bhowmik Reference McElroy, Bhowmik, Stier, Horgan and Bonos2013; USEPA 2010). It is an ionizable herbicide [acid dissociation constant (pK a) = 3.5; weak acid] with low water solubility [solubility constant (K s) = 2.04 g L−1; pH 7, 25 C] and moderate sorption to organic carbon [organic carbon water partition coefficient (K oc) = 434 to 1,544 mL g−1], which acts by inhibiting cellulose biosynthesis, thereby representing a unique mode of action for selective and broad-spectrum weed control in select crop areas and turf (Jeffries and Gannon Reference Jeffries and Gannon2016; Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastiaon and Nissen2017a; Shaner Reference Shaner2014a; USEPA 2010). Oxadiazon is another common preemergence herbicide used in select turfgrass and non-cropland areas (Shaner et al. Reference Shaner2014b). Oxadiazon is a non-ionizable herbicide successful in preemergence weed control as a result of its moderate to long-term field persistence (average T 1/2 = 60 d), low aqueous solubility (K s = 0.7 mg L−1; 20 C), and high affinity for soil organic matter (K oc = 1,409 to 3,268 mL g−1) (Lewis et al. Reference Lewis, Tzilivakis, Warner and Green2016; Shaner Reference Shaner2014b; USEPA 2003). Evidence suggests that preemergence applications of indaziflam at up to 55 g ha−1 or oxadiazon at up to 4,400 g ha−1 before the beginning of spring may provide over 80% control of summer annual weeds such as goosegrass, smooth crabgrass [Digitaria ischaemum (Schreb.) Schreb. Ex Muhl.], large crabgrass, annual sedge (Cyperus compressus L.), and Florida pusley (Richardia scabra L.) (Brosnan et al. Reference Brosnan, McCullough and Breedan2011, Reference Brosnan, Breeden, McCullough and Henry2012; Brecke et al. Reference Brecke, Stephenson and Unruh2010; Toler et al. Reference Toler, McCarty and Higingbottom2003). Perry et al. (Reference Perry, McElroy, Doroh and Walker2011) showed that indaziflam can provide >90% large-crabgrass control extending 29 wk after treatment (WAT) after a single application at 60 g ha−1 whereas Johnson and Murphy (Reference Johnson and Murphy1996) observed 97% control of the same species 24 WAT with a single oxadiazon application at 4,483 g ha−1. However, these studies only evaluated weed injury or control using visual assessment, and pertinent information regarding indaziflam or oxadiazon residue concentration required for acceptable weed suppression throughout the weed emergence period is currently unavailable. In a greenhouse study by McElroy et al. (Reference McElroy, Head, Wehtje and Spak2017), oxadiazon at 1,120 g ha−1 completely prevented goosegrass seedling emergence, and indaziflam at 30 g ha−1 prevented seedling emergence and aboveground biomass accumulation 42 DAT. However, as herbicide effective concentrations for seedling emergence inhibition and phytotoxicity in summer annual weeds or control of perennial weed species relevant to southeastern US turf systems remain unknown, managing such weed species common to the region remains challenging.
Barnyardgrass is a summer annual grass commonly found in moist cultivated turfgrass systems that grows prostrate and exhibits prolific reproduction by seed (Uva et al. Reference Uva, Neal and DiTomaso1997). Broadleaf signalgrass is a summer annual grass that is competitive in cultivated or disturbed sandy areas and is highly adaptable, making it difficult to suppress (Burke et al. Reference Burke, Thomas, Spears and Wilcut2003; Johnson and Coble Reference Johnson and Coble1986). Doveweed is a summer annual broadleaf that is difficult to control in cultivated turf as it reproduces by seeds and stolons and grows rapidly to form dense stands that compete with desirable turf species for light and nutrients (Atkinson Reference Atkinson2014). Large crabgrass is a summer annual that is abundantly stoloniferous and well-adapted to humid and semi-arid south and southeastern United States (Dalrymple et al. Reference Dalrymple, Mitchell, Flatt, Dobbs, Ingram and Coleman1999). Purple nutsedge is a perennial sedge with a history of invasiveness that most commonly reproduces through tubers and grows in damp or poorly drained soils in the southern United States (USFWS 2015; Uva et al. Reference Uva, Neal and DiTomaso1997). The performance of preemergence herbicides in controlling these weed species often depends on the length of their efficacy coordinated with the length of the weed emergence period for consistent weed control throughout the growing season (Johnson and Murphy Reference Johnson and Murphy1996). Although some preemergence herbicides, including indaziflam and oxadiazon, possess postemergence activity, they should be applied before germination and emergence to obtain acceptable results in summer annual weeds. Although optimum application time may vary widely with geography, it is generally guided by mean soil temperatures based on temperature optima for specific weed species (Clark and Kenna Reference Clark, Kenna, Krieger and Krieger2001; Watschke et al. Reference Watschke, Dernoeden and Shetlar2013). More research is required to quantify the critical concentrations necessary for preemergence control to assess indaziflam and oxadiazon use parameters for adequate long-term control. Bioassay methods are powerful tools for measuring response to soil herbicide residue, because they capture total residual phytotoxicity and provide qualitative data for effective herbicide concentration estimation (Jeffries and Gannon Reference Jeffries and Gannon2016; Sandín-España et al. Reference Sandín-España, Loureiro, Concepcion, Cristina, Santìn-Montanya, Soloneski and Larramendy2011; Streibig and Kudsk Reference Streibig and Kudsk1993). Bioassay experiments conducted with winter annual weeds indicate that 0.19 and 0.56 g ha−1 indaziflam provide 50% reduction in dry biomass of downy brome (Centaurea diffusa Lam.) and feral rye (Secale cereale L.), respectively, and 0.06 to 1.33 g ha−1 provide 50% dry-biomass reduction in six invasive winter annual grass species 4 to 5 WAT (Sebastian et al. Reference Sebastian, Nissen, Scott and De Souza Rodrigues2016, Reference Sebastian, Nissen, Sebastian, Meiman and Beck2017b). However, a knowledge gap exists for the response of summer annuals common to turfgrass systems to indaziflam and oxadiazon.
The objectives of this research were to (i) quantify indaziflam and oxadiazon effective concentrations for seedling emergence inhibition and control of shoot and root biomass in barnyardgrass, broadleaf signalgrass, doveweed, large crabgrass, and purple nutsedge; (ii) determine the effectiveness of indaziflam and oxadiazon in controlling selected weed species throughout their specific periods of emergence. This research was initiated as the first step toward evaluating whether the current prescribed application timings and rates and number of applications for indaziflam and oxadiazon are adequate for season-long control of the weeds of interest. Generation of such information can help guide the development of preemergence herbicide programs targeted toward emergence periods and optimum soil temperatures for seedling emergence for efficacious long-term, species-specific control.
Materials and Methods
Greenhouse Bioassays
Greenhouse bioassays were conducted using five weed species to quantify the effective indaziflam or oxadiazon concentration required for 50% (EC50), 80% (EC80), and 90% (EC90) inhibition of seedling emergence and shoot and root mass reduction. Bioassays were initiated in the greenhouse (North Carolina State University Method Road Greenhouse Facilities, Raleigh, NC). Barnyardgrass, broadleaf signalgrass, doveweed, and large crabgrass seeds and purple nutsedge tubers were either collected from turfgrass research fields located in North Carolina or purchased (Azlin Seed Service, Leland, MS) and stored in a freezer at −4 C until planting. Based on preliminary laboratory germination tests of each species, 13 seeds of barnyardgrass or doveweed and 14 seeds of large crabgrass or signalgrass were planted at 0.5 cm depth in individual 10 by 10 by 9 cm plastic pots (0.5 L) containing 300 g sand. Seedlings were thinned 1 d after seedling emergence to achieve a target density of 11 plants per pot, and a single tuber of purple nutsedge was planted similarly (100% viability). All species were planted 1 d prior to herbicide application, and the soil surface was smoothed and irrigated after planting.
Herbicide treatments included indaziflam (Specticle® FLO; Bayer Environmental Science, Cary, NC) at 0, 4, 8, 12, 17, 21, 25, 29, 33, and 37 g ha−1 or oxadiazon (Ronstar® FLO; Bayer Environmental Science, Cary, NC) at 0, 420, 841, 1,260, 1,681, 2,102, 2,354, 2,942, 3,363 and 3,783 g ha−1. Indaziflam and oxadiazon rates were selected as 0, 12.5%, 25%, 37.5%, 50%, 62.5%, 75%, 87.5%, 100%, and 112.5% of 33 and 3,363 g ha−1, respectively—the regionally accepted single application rate for summer annual weed control. A single herbicide dose of indaziflam or oxadiazon was applied to replicated pots using a CO2-pressurized backpack sprayer calibrated to deliver 304 L ha−1 and equipped with a XR11003 nozzle (TeeJet®, Spraying Systems Co., Wheaton, IL).
After herbicide application, pots were maintained in the greenhouse with day/night average temperatures of 25/18 C and 60% relative humidity. High-intensity supplemental lighting was provided to extend the photoperiod to 14 h. Each pot was irrigated daily with approximately 30 mL water to maintain optimal soil moisture for growth while caution was taken to prevent herbicide leaching. After seedling emergence, plants were fertilized weekly to provide the equivalent of 12 kg N ha−1 (Peters Professional® 20–20–20 Water Soluble Fertilizer; Scotts-Sierra Horticultural Products Co., Marysville, OH). The experiment included nine herbicide treatments and nontreated controls replicated four times and arranged in a completely randomized design and was conducted twice.
Data Collection and Statistical Analyses
Emerged seedling counts of barnyardgrass, broadleaf signalgrass, doveweed, and large crabgrass in treated and nontreated pots were monitored weekly and recorded. Emerging shoot counts from purple nutsedge tubers were monitored similarly. The maximum number of emerged seedlings was observed 14 DAT; therefore, values from this timing were used for analysis. Seedling or tuber emergence data were converted to percent seedling emergence inhibition compared to counts in nontreated pots (where all weed species exhibited 100% seedling emergence) using the following equation:
Plants were harvested from pots 84 DAT, and aboveground vegetation (henceforth referred to as shoot) was separated from belowground vegetation (root), washed to eliminate soil debris, weighed, fresh mass recorded, and then was placed into paper bags. Root and shoot vegetation were dried in an oven at 60 C for 96 h, and dry mass was recorded. Fresh and dry mass were converted to percent reduction compared to the nontreated using the equation:
where NT and T are from nontreated and treated, respectively.
Percent seedling emergence inhibition and reduction in fresh and dry shoot and root mass were subjected to ANOVA using PROC MIXED in SAS (SAS/STAT 9.4, SAS Institute, Cary, NC) to test for significance of main effects and interactions at the 0.05 level of probability. Experimental run, weed species, and herbicide rate were considered fixed effects, whereas replication was considered a random effect. The main effect of weed species and interactions were significant at α = 0.05; therefore, data were sorted by weed species using PROC SORT. Where the effect of herbicide rate was found to be significant with P values ≤ 0.05, the data were further analyzed using nonlinear regression to describe the response of weed species to indaziflam and oxadiazon. Data were pooled across the two experimental runs where the main effect and interactions of run were not significant (α = 0.05).
A four-parameter log-logistic response curve was utilized to describe the relationship between the measured plant response (y) and the logarithm of the herbicide dose (x) using the equation:
where EC 50 denotes the dose required for halfway response between the lower limit C and upper limit D, and B represents the Hill slope around the EC 50 (Motulsky and Christopoulos Reference Motulsky and Christopoulos2003; Sebastian et al. Reference Sebastian, Nissen, Sebastian, Meiman and Beck2017b). Curve fitting was performed using nonlinear regression in GraphPad Prism (version 8.0.0 for Windows; GraphPad Software, San Diego, CA). The lower limit of the curve was constrained to 0, and upper limit of the curve was constrained to 100 since mean response in nontreated plants was never lower than 0%, and the mean maximum response did not exceed 100% on the scale used. Any one dose-response level can be expressed by a function of the parameters B and EC 50 , in that:
where EC x represents the effective dose that elicits x% response between the upper and lower limits of the curve (Zhang et al. Reference Zhang, McGiffen, Becker, Ohr, Sims and Kallenbach1997). This equation was used to calculate the effective herbicide concentration that elicited 80% (EC80) and 90% (EC90) response.
Additionally, 20-yr monthly mean soil temperature at Lake Wheeler Turfgrass Research Lab (North Carolina State University, Raleigh, NC) (35.73001 N, 78.68758 W), representative of the southeastern United States, were compared with the published soil temperature(s) required for germination to identify the periodicity of emergence for barnyardgrass, broadleaf signalgrass, doveweed, large crabgrass, and purple nutsedge. Temperature data were accessed using CRONOS (State Climate Office of North Carolina, NC State University, Raleigh, NC). Assuming an application of indaziflam and oxadiazon at the regionally accepted single application rate (33 or 3,363 g ha−1, respectively) in mid-March before the start of spring (approximately March 20 in the region), distribution in soil up to a depth of 5.1 and 2.5 cm, respectively, and published minimum half-life values of 150 and 60 d, respectively, indaziflam or oxadiazon concentration remaining during the individual species-specific emergence periods was predicted using the following first-order rate of degradation and half-life equations:
and
where [A] is the herbicide concentration remaining (g ha−1) at time t (d), [A]0 is the initial herbicide concentration at the applied rate (g ha−1), k is the first-order degradation rate constant, and T 1/2 is the half-life or time required for 50% of the initial concentration of herbicide to be degraded (d) (Jhala et al. Reference Jhala, Ramirez and Singh2013; Kah et al. Reference Kah, Beulke and Brown2007; Shaner Reference Shaner2014a, Reference Shaner2014b).
Results and Discussion
Data from the two experimental runs were pooled, because main effect and interactions of run were not significant (P > 0.05). ANOVA revealed significant effect of herbicide rate on percent seedling emergence inhibition (P < 0.001) in barnyardgrass, broadleaf signalgrass, doveweed, and large crabgrass 14 DAT; therefore, percent seedling emergence inhibition for these weed species was further analyzed using nonlinear regression fit to the log-logistic model to describe the response to indaziflam and oxadiazon. Emergence of purple nutsedge tubers was not inhibited by indaziflam or oxadiazon at any evaluated rate throughout the duration of the experiment; therefore, these data were excluded from curve-fitting procedures. The effect of herbicide rate was significant for percent shoot and root mass reduction (P < 0.001) in all evaluated weed species, and these data were further analyzed using nonlinear regression fit to the log-logistic model. Additionally, the Hill slope was constrained for the dose–response curves describing shoot and root mass reduction in doveweed by indaziflam to enable a stable curve-fit (Table 1) (Motulsky and Christopoulos Reference Motulsky and Christopoulos2003). The results from regression analyses and comparisons to predicted herbicide concentrations are presented and discussed.
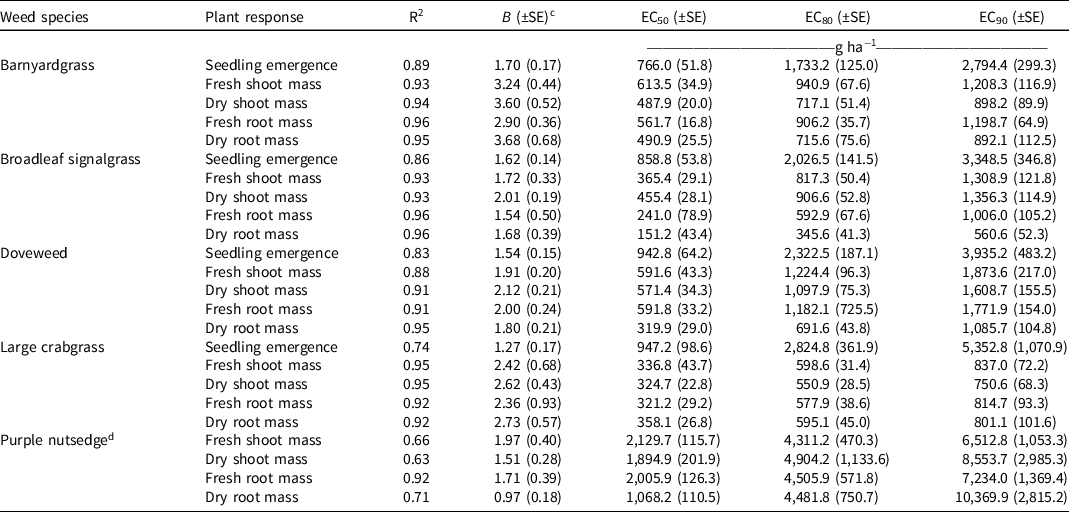
Table 1. Coefficient of determination (R2), Hill slope (B), and indaziflam concentration (g ha−1) that provided 50% (EC50), 80% (EC80), and 90% (EC90) seedling emergence inhibition 14 d after treatment (DAT) and fresh and dry shoot and root mass reduction 84 DAT in five weed species. a, b

a Values were calculated from nonlinear regression equations for log-logistic dose–response curves (Equations 3 and 4).
b No new emergence was observed beyond 14 d in treated or nontreated pots.
c Values in parentheses are standard errors.
d Standard errors are absent because Hill slope (B) was constrained to the shown value for the best-fitting curve fit.
e R2 and regression parameters are absent for seedling emergence inhibition in purple nutsedge, as emerging shoot growth from tubers was not inhibited by any evaluated indaziflam rate; therefore, data were excluded from regression analysis.
Barnyardgrass
Indaziflam EC50 and EC80 for barnyardgrass seedling emergence inhibition 14 DAT was 6.9 and 20.1 g ha−1, respectively (Table 1). However, EC90 (37.7 g ha−1) for a similar response was higher than the prescribed application rate evaluated in this research (33 g ha−1). EC50, EC80, and EC90 for fresh shoot and root mass reduction 84 DAT ranged from 1.2 to 10.0 g ha−1, whereas indaziflam concentrations for similar levels of dry shoot and root mass reduction ranged from 1.0 to 7.4 g ha−1, with dry-mass reduction requiring lower concentration. Results from the current study correspond with previous research where indaziflam applied at 36.5 g ha−1 resulted in >95% barnyardgrass control 84 DAT in an orchard growth environment (González-Delgado et al. Reference González-Delgado, Shukla, Ashigh and Perkins2016).
Oxadiazon EC50, EC80, and EC90 for barnyardgrass seedling emergence inhibition 14 DAT ranged from 766.0 to 2,794.4 g ha−1 and were below the prescribed single application rate of 3,363 g ha−1 (Table 2). EC90 results for dry shoot and root mass reduction were 892.1 and 898.2 g ha−1, respectively, whereas EC90 for fresh shoot and root mass reduction was approximately 1,200 g ha−1. Of the evaluated rates, indaziflam >25 g ha−1 and oxadiazon >2,942 g ha−1 resulted in 100% reduction of above and belowground biomass of emerged barnyardgrass 84 DAT (Figures 1 and 2).
Table 2. Coefficient of determination (R2), Hill slope (B), and oxadiazon concentration (g ha−1) that provided 50% (EC50), 80% (EC80), and 90% (EC90) seedling emergence inhibition 14 d after treatment (DAT) and fresh and dry shoot and root mass reduction 84 DAT in five weed species. a, b
a Values were calculated from nonlinear regression equations for log-logistic dose–response curves (Equations 3 and 4).
b No new emergence was observed beyond 14 d in treated or nontreated pots.
c Values in parentheses are standard errors.
d R2 and regression parameters are absent for seedling emergence inhibition in purple nutsedge, as tuber seedling emergence was not inhibited by any evaluated indaziflam rate; therefore, data were excluded from regression analysis.
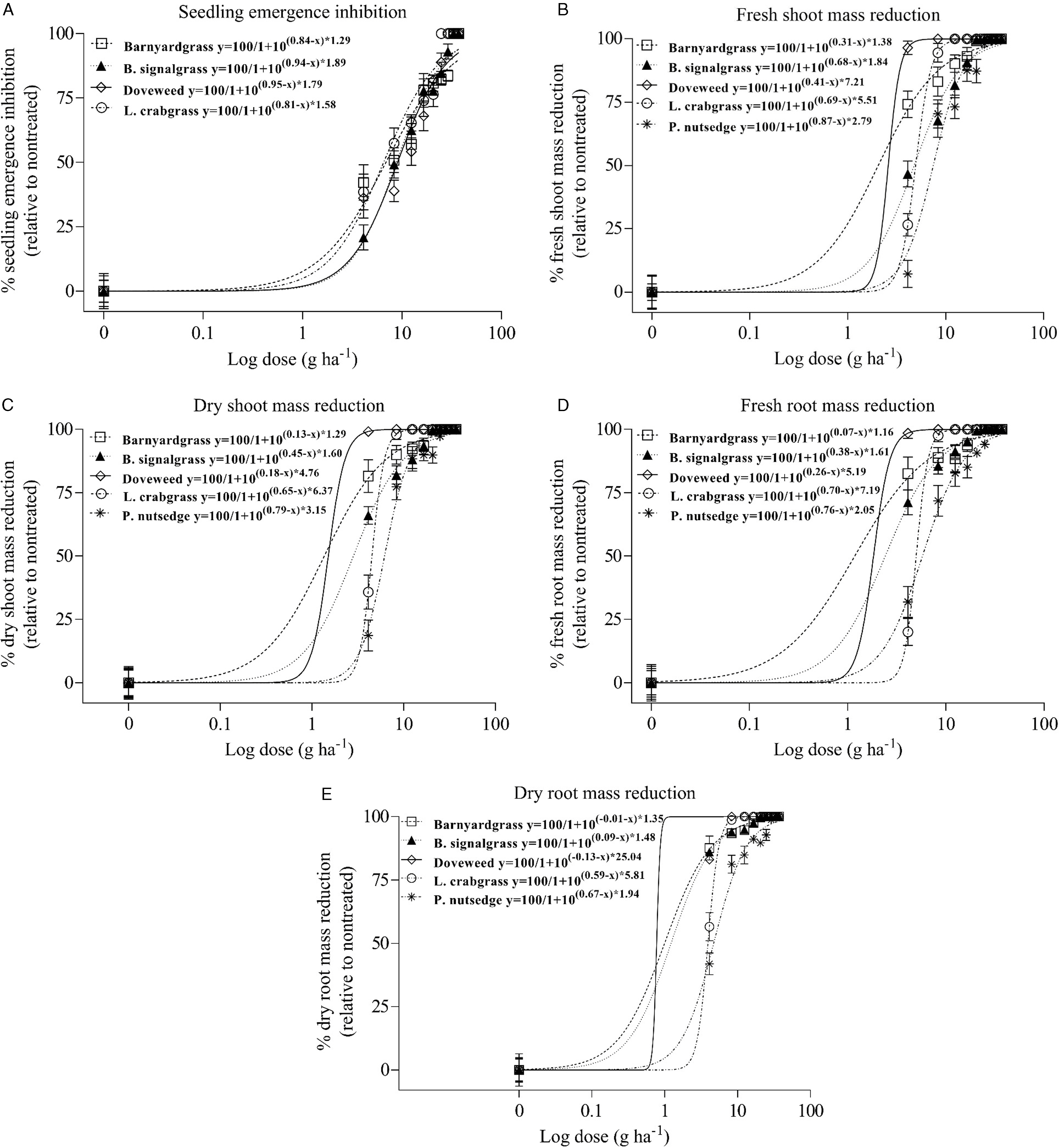
Figure 1. Seedling emergence inhibition (A), fresh shoot mass reduction (B), dry shoot mass reduction (C) fresh root mass reduction (D), and dry root mass reduction (E) in barnyardgrass, broadleaf signalgrass (B. signalgrass), doveweed, large crabgrass (L. crabgrass), and purple nutsedge (P. nutsedge) to indaziflam. Vertical bars represent ± SE of means (n = 8). Dose–response curves were fit to a log-logistic model using nonlinear regression (Equation 3).
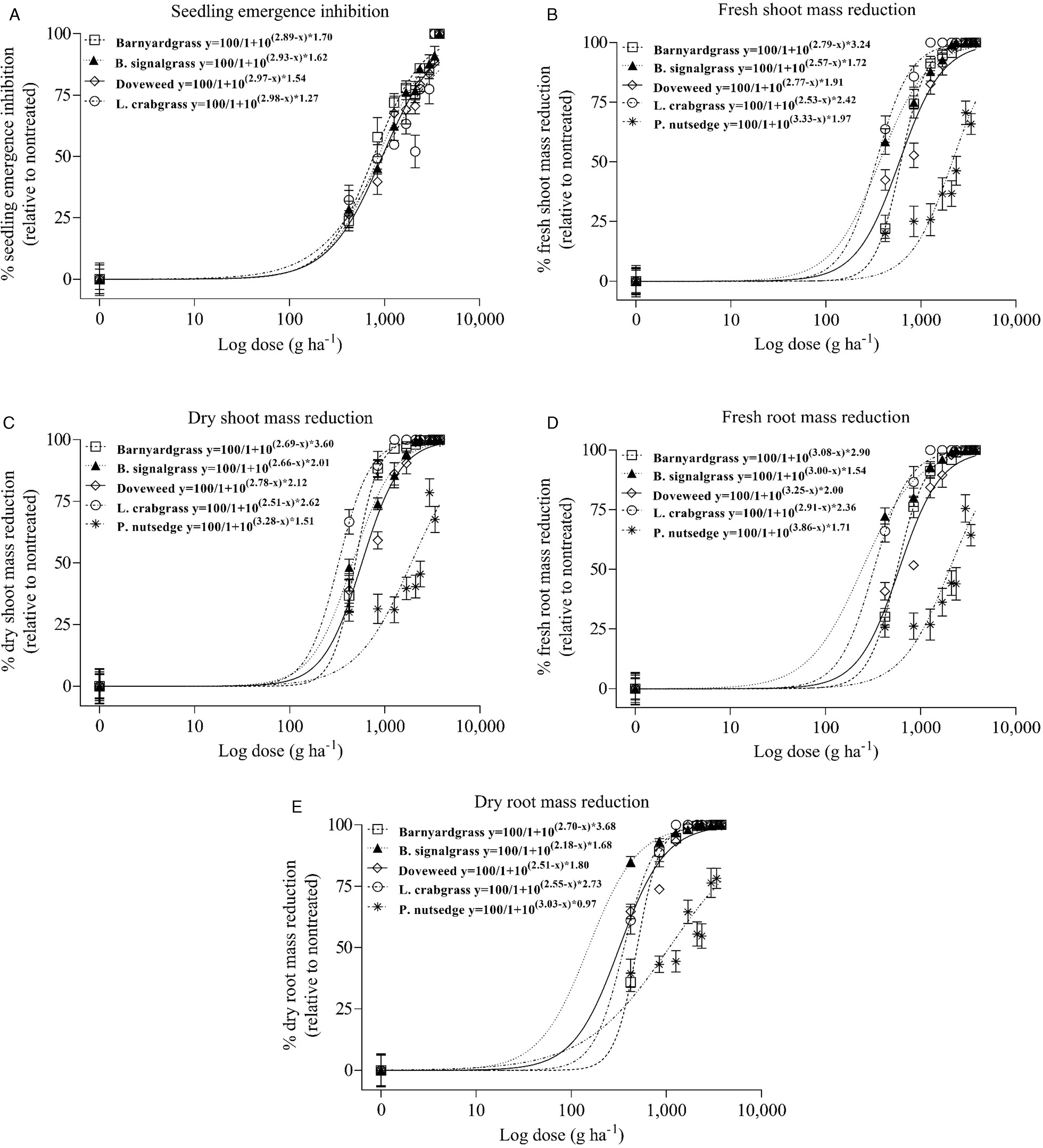
Figure 2. Seedling emergence inhibition (A), fresh shoot mass reduction (B), dry shoot mass reduction (C), fresh root mass reduction (D), and dry root mass reduction (E) in barnyardgrass, broadleaf signalgrass (B. signalgrass), doveweed, large crabgrass (L. crabgrass), and purple nutsedge (P. nutsedge) to oxadiazon. Vertical bars represent ± SE of means (n = 8). Dose–response curves were fit to a log-logistic model using nonlinear regression (Equation 3).
Barnyardgrass seeds germinate from early spring to midsummer when the soil reaches a temperature between 15 and 18 C (Watschke et al. Reference Watschke, Dernoeden and Shetlar2013). This soil temperature range corresponds to a periodicity of emergence that ranges from April to mid-July in Raleigh, NC (Figure 3 and Table 3). Indaziflam concentration required for 90% emergence inhibition may not be adequately maintained throughout the period of barnyardgrass emergence when indaziflam is applied before the beginning of spring at 33 g ha−1 based on predicted residue concentration (Tables 1 and 3). The indaziflam concentration required for 80% seedling emergence inhibition may not be present by mid-July, when soil temperatures may still be suitable for barnyardgrass germination and emergence. However, it is likely that indaziflam residue concentration will be maintained during the growing season to control up to 90% root and shoot mass in plants that may have escaped an indaziflam preemergence application.

Figure 3. Daily and monthly mean 20-yr (2000–2020) soil temperature profile for Lake Wheeler Turfgrass Field Lab (North Carolina State University, Raleigh, NC), accessed from CRONOS.
Table 3. Predicted indaziflam and oxadiazon concentrations (g ha−1 and mg kg−1) during periodicity of emergence of selected weed species.
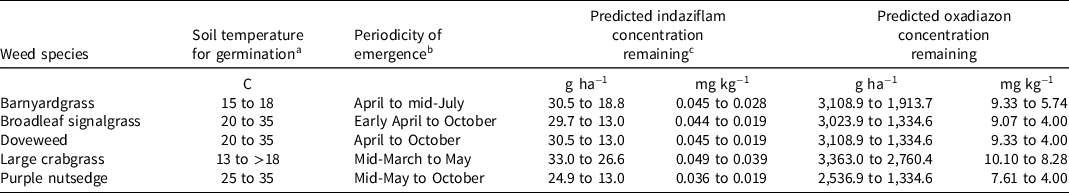
a Soil temperature values from published literature: Burke et al. Reference Burke, Thomas, Spears and Wilcut2003; Nishimoto Reference Nishimoto2001; Watschke et al. Reference Watschke, Dernoeden and Shetlar2013; and Wilson et al. Reference Wilson, Burton, Spears and York2006.
b Periodicity of emergence in Raleigh, NC, identified by comparing soil temperatures for germination with 20-yr monthly mean soil temperatures at Lake Wheeler Turfgrass Field Lab, Raleigh, NC, accessed from CRONOS (State Climate Office of North Carolina, NC State University, Raleigh, NC).
c Predicted indaziflam and oxadiazon concentrations during respective periodicity of emergence of each weed species calculated using Equations 5 and 6. Assumptions include indaziflam and oxadiazon applied on March 15 (i.e., before March 20—beginning of spring) at the regionally accepted single application rate of 33 or 3,363 g ha−1, respectively, distribution in soil up to a depth of 5.1 and 2.5 cm, respectively, and half-life of 150 and 60 d, respectively, as per published literature (Anonymous 2016, 2020; Shaner Reference Shaner2014a, Reference Shaner2014b).
When oxadiazon is applied before the start of spring at 3,363 g ha−1, the predicted concentration range indicates that 50% to 80% emergence inhibition may be achievable during the period of barnyardgrass emergence, but the concentration for 90% emergence inhibition may not be maintained by mid-July (Tables 2 and 3). Like indaziflam, 50% to 90% control of shoot and root mass may be feasible throughout the emergence season of this species by oxadiazon from a single preemergence application at the prescribed rate and timing. Results from this study also show that barnyardgrass is susceptible to oxadiazon at a normal use rate, as previously indicated in studies conducted in rice systems (Chin Reference Chin2001).
Broadleaf Signalgrass
Indaziflam and oxadiazon >21 g ha−1 and >2,354 g ha−1, respectively, reduced shoot and root mass by 100% in broadleaf signalgrass 84 DAT compared to nontreated (Figures 1 and 2). Indaziflam EC50 and EC90 for seedling emergence inhibition 14 DAT were 8.8 and 28.0 g ha−1, respectively, and the concentrations for 50% and 80% biomass reduction ranged from 1.2 to 4.8 g ha−1 and 5.4 to 15.7 g ha−1, respectively (Table 1). EC50 and EC90 for seedling emergence inhibition by oxadiazon 14 DAT were 858.8 and 3,348.5 g ha−1, respectively, and the oxadiazon concentration required for similar levels of dry root mass reduction 84 DAT were approximately six times lower (Table 2).
Broadleaf signalgrass is highly propagated by seed, and Burke et al. (Reference Burke, Thomas, Spears and Wilcut2003) found that seed germination occurs between 20 and 35 C. Indaziflam applied in mid-March at the prescribed application rate may likely provide season-long seedling emergence inhibition and biomass control, even though residue concentration may decrease by the end of the emergence season to a concentration that is lower than required for 90% seedling emergence inhibition (Tables 1 and 3). Oxadiazon from a single application in mid-March at a rate of 3,363 g ha−1 may be efficacious for only 50% to 80% inhibition of seedling emergence and shoot mass reduction (Tables 2 and 3). Further investigation of management techniques for broadleaf signalgrass is necessary, as this weed is easily adaptable to different growing environments, which enables it to become more prevalent and competitive. Research has elucidated the effects of early-postemergence and postemergence herbicides on broadleaf signalgrass, but it is advantageous to examine the efficacy of preemergence herbicides with alternate modes of action to prevent further development of herbicide tolerance in this species, of which numerous cases are already documented (Burke et al. Reference Burke, Thomas, Spears and Wilcut2003; Johnston and Coble Reference Johnson and Coble1986).
Doveweed
Indaziflam concentrations required for 50%, 80%, and 90% doveweed seedling emergence inhibition 14 DAT ranged from 9.0 to 30.8 g ha−1—lower than the prescribed rate for a single application (Table 1). Indaziflam concentration for shoot and root mass reduction 84 DAT ranged between 0.7 and 2.6 g ha−1 for 50% and from 0.8 to 3.5 g ha−1 for 90% reduction. Oxadiazon EC50 and EC80 were 942.8 and 2,322.5 g ha−1, respectively, for seedling emergence inhibition 14 DAT, whereas EC90 was 3,935.2 g ha−1 and higher than the prescribed application rate (Table 2). Oxadiazon concentrations for 50% fresh shoot mass and root mass reduction 84 DAT were similar, whereas the concentration for dry root mass reduction was lower. Atkinson (Reference Atkinson2014) reported that 54 g ha−1 indaziflam and 3,360 g ha−1 oxadiazon in March provide approximately 28% and 24% doveweed control, respectively, 112 DAT. However, we found that indaziflam ≥29 g ha−1 and 3,363 g ha−1 oxadiazon cause 100% and 88% seedling emergence inhibition, respectively, 14 DAT with no new emergence recorded up to 84 DAT (Figures 1 and 2).
Atkinson (Reference Atkinson2014) postulated that the elapsed time between herbicide application (mid-March) and germination in June was “likely too long to provide preemergence control throughout the peak doveweed germination season”. Therefore, higher inhibition of emergence may have been exhibited in the present bioassay experiments, because the time between herbicide application and seed planting was 1 d. It may also be hypothesized that herbicide persistence may have been shorter under variable conditions of the field environment as per the study by Atkinson (Reference Atkinson2014) compared to greenhouse conditions utilized in the present research, resulting in higher soil residual activity and efficacy.
Doveweed seeds germinate between 20 and 35 C soil temperature, which aligns with an April to October periodicity of emergence in Raleigh, NC (Figure 3 and Table 3) (Wilson et al. Reference Wilson, Burton, Spears and York2006). Indaziflam, when applied as prescribed, may be adequate for 50% seedling emergence inhibition and up to 90% shoot and root mass reduction throughout the growing season (Tables 1 and 3). However, indaziflam concentrations for 80% and 90% seedling emergence inhibition may not be maintained during the emergence period. Oxadiazon applied in early spring at the prescribed rate may provide up to 80% emergence inhibition during the emergence season, and 80% biomass control (Tables 2 and 3). However, oxadiazon concentration for up to 90% root mass reduction may be adequately maintained, thus suppressing doveweed establishment after emergence.
Large Crabgrass
Complete plant death was observed from indaziflam ≥12 g ha−1 and oxadiazon ≥1,260 g ha−1, indicating that large crabgrass is sensitive to indaziflam and oxadiazon (Figures 1 and 2). Indaziflam EC80 and EC90 results for seedling emergence inhibition 14 DAT were 15.7 and 26.3 g ha−1, respectively, and relatively higher concentrations were quantified for similar percentages of fresh or dry shoot and root mass reduction 84 DAT (Table 1). Oxadiazon concentration for 50% to 90% seedling emergence inhibition 14 DAT varied from 947.2 to 5,352.8 g ha−1, and the concentrations for 50%, 80%, and 90% fresh or dry shoot and root mass reduction 84 DAT were ≥ 321.2, 550.9, and 750.6 g ha−1, respectively (Table 2).
The soil temperature reported for crabgrass germination is above 13 C, whereas other reports state that soil temperatures above 18 C support highest germination (Watschke et al. Reference Watschke, Dernoeden and Shetlar2013). When applied at the prescribed rate in mid-March, indaziflam may successfully control seedling emergence and shoot and root biomass by up to 90%, and oxadiazon may suppress up to 80% seedling emergence and up to 90% shoot and root accumulation until May (Tables 1 to 3). Most preemergence herbicide research to date has been conducted with other crabgrass species; therefore, future studies utilizing large crabgrass to generate more data regarding effective herbicide concentrations for its control and to test herbicide efficacy under field conditions would be beneficial to develop better chemical management programs for this species.
Purple Nutsedge
Indaziflam >29 g ha−1 caused complete plant death in purple nutsedge 84 DAT (Figure 1). Indaziflam was efficacious in reducing purple nutsedge shoot and root mass by 90% at 12.4 and 14.6 g ha−1, respectively, 84 DAT (Table 1). Oxadiazon did not cause complete plant death in purple nutsedge at any evaluated rate. Up to 8,553.7 and 10,369.9 g ha−1 were quantified as necessary for 90% fresh and dry shoot and root mass reduction, respectively, which are >2.5 times the prescribed application rate (Figure 2 and Table 2). Neither indaziflam nor oxadiazon inhibited purple nutsedge emergence during the entirety of the experiment. While S-metolachlor, sulfentrazone, MSMA, halosulfuron, imazaquin, and imazapic have been found to reduce the viability of purple nutsedge tubers by at least 40% in bare-ground sites, sequential or early-postemergence herbicide applications may be necessary, and additional research is required in established turfgrass systems (Blum et al. Reference Blum, Isgrigg and Yelverton2000; Brecke et al. Reference Brecke, Stephenson and Unruh2005; Webster et al. Reference Webster, Grey and Ferrell2017).
Purple nutsedge tubers tend to break dormancy when soil temperature is above 15 C, with optimum sprouting between 25 and 35 C (Nishimoto Reference Nishimoto2001). Therefore, the periodicity of purple nutsedge emergence in Raleigh, NC, is likely to extend from mid-May to October (Figure 3 and Table 3). Based on the predicted indaziflam concentration during this period under select assumptions, 50% to 80% control of purple nutsedge may be achieved during the peak germination and emergence season, and 90% biomass control may be achieved until the end of August (Tables 1 and 3). Up to 50% control of shoot and root mass may be feasible by oxadiazon at the prescribed application rate and timing (Tables 2 and 3).
In conclusion, indaziflam and oxadiazon are successful in controlling summer annual weed emergence, likely owing to innate species sensitivity and specific herbicide persistence. Factors that influence herbicide efficacy include herbicide rate, application timing, and bioavailability in soil, soil texture, organic matter content and pH, temperature, moisture, microbial populations, and climatic conditions (Cox et al. Reference Cox, Hermosin and Cornejo1995; Jeffries and Gannon Reference Jeffries and Gannon2016; Swanton and Weise Reference Swanton and Weise1991). Though findings of this research are limited to the specific soil type and annual and perennial weeds investigated in this study, further research should include field efficacy trials with weed species relevant to southeastern United States to fully evaluate the extent of weed control by indaziflam and oxadiazon in turfgrass systems.
Practical Implications
These research findings show that seedling emergence inhibition may require a higher indaziflam or oxadiazon concentration than shoot or root biomass reduction among the evaluated weed species. This indicates that application timing and rate are critical for effective preemergence control of seedling emergence. Turfgrass managers may opt for a split application of indaziflam or oxadiazon during the periodicity of weed emergence at appropriately adjusted application rates and timings to provide a higher level of control, especially for controlling weeds that may escape a single preemergence application. Split applications that better correspond with the optimum soil temperature for germination of specific species could ensure that emerging weeds are exposed to lethal herbicide residue throughout their respective optimal germination periods. Turfgrass managers can select application timings and rates based on weed emergence periods and herbicide efficacy that correspond to region-specific soil temperature variations and soil type and texture. Future research under field conditions in varying soil types can further evaluate indaziflam and oxadiazon efficacy and optimal application rates for adequate control of diverse weed species throughout their emergence and growth periods.
Acknowledgments
The authors would like to thank Matheus Teixeira and Scott Brinton for their work toward this research. No conflicts of interest have been declared.











