Introduction
Black nightshade (Solanum nigrum L.) is native to Eurasia (western Europe to Japan) and is widely distributed throughout the P. R. China. It is a troublesome weed that spreads mainly by seed dispersal (Bravo et al. Reference Bravo, Velilla, Bautista and Peco2014; Suthar et al. Reference Suthar, Naik and Mulani2009). A single plant can produce up to 600,000 seeds and build a huge seedbank in the soil, which are difficult to prevent and control, mostly in corn (Zea mays L.) and cotton (Gossypium hirsutum L.) fields (Kremer and Kropff Reference Kremer and Kropff1998; Li Reference Li2017; Sarkinen et al. Reference Sarkinen, Poczai, Barboza, Van der Weerden, Baden and Knapp2018). The competitiveness of weeds is strongly influenced by habitat conditions (Defelice Reference Defelice2003). Temperature is the most important factor affecting weed seed germination (Malavert et al. Reference Malavert, Batlla and Benech-Arnold2020). The cumulative germinability of seeds differs over time under different temperature conditions, and the germination reaches a maximum at a constant temperature. For a specific weed population, the germination response to temperature is a long-term adaptation to its growth environment. To obtain more insights into the response of seed germination to temperature and the timing of seedling emergence in the field, it is necessary to understand how temperature affects the seed dormancy and germination of S. nigrum (Taab and Andersson Reference Taab and Andersson2009).
Seed germination is influenced by three fundamental temperature points: base temperature (T b, the lowest temperature at which seeds can germinate), optimum temperature (T o, the temperature at which the germination is most rapid), and ceiling temperature (T c, the highest temperature at which germination can occur) (Batlla and Benech-Arnold Reference Batlla and Benech-Arnold2015; Bewley et al. Reference Bewley, Bradford, Hilhorst and Nonogaki2013). Previous studies showed that base temperature and ceiling temperature for the germination of S. nigrum populations from Sweden were 18 and 34 C, respectively (Taab and Andersson Reference Taab and Andersson2009). For S. nigrum populations from Hebei in China, the constant temperature range for seed germination is between 15 and 30 C (Dong et al. Reference Dong, Ma, Wu, Jiang and Ma2020). The optimum temperature range for germination of S. nigrum for populations from India was 26 to 30 C (Suthar et al. Reference Suthar, Naik and Mulani2009). However, Givelberg and Horowitz (Reference Givelberg and Horowitz1984) showed that the base temperature for the germination of S. nigrum seeds collected in Israel was 20 C, the ceiling temperature was 35 C, and the optimum temperature range was 25 to 30 C. In this context, S. nigrum seeds of different geographic origin exhibited variation in germination response to temperature, which may reflect local adaptation or the adaptive potential to respond to climatic conditions.
Hydrothermal time models were developed to analyze the effects of temperature and water potential on seed germination of many weed species (Alm et al. Reference Alm, Stoller and Wax1993; Guillemin et al. Reference Guillemin, Gardarin, Granger, Reibel, Munier-Jolain and Colbach2013). While seed germination is highly linked to temperature, a thermal time model can better describe seed germination in response to temperature (Arana et al. Reference Arana, Gonzalez-Polo, Martinez-Meier, Gallo, Benech-Arnold, Sánchez and Batlla2016; Carhuancho León et al. Reference Carhuancho León, Aguado Cortijo, Morató Izquierdo and Castellanos Moncho2020; Saffariha et al. Reference Saffariha, Jahani and Potter2020). These models allow a comparison of the differences in response to environmental temperature during seed germination by comparing differences in the base temperature and accumulated growing degree days (GDD) among populations, quantification of seed dormancy, and the prediction of the time to seedling emergence. Understanding the germination response of S. nigrum to environmental factors, primarily temperature, and related approaches to weed management will provide a broader perspective for comprehensive and effective management of weeds.
The purpose of this study was to investigate seed germination response to temperature in six populations of S. nigrum from different climatic regions of China; to simulate and predict the seed germination using a thermal time model combined with a logistic function to explore whether the fundamental assumptions of the thermal time model are applicable to seed germination of S. nigrum; and to predict the base temperature, optimum temperature, ceiling temperature, and accumulated GDD required for seed germination in each population.
Materials and Methods
Plant Material
Solanum nigrum seeds were collected in two consecutive years (August 2018 and August 2019) from six mid to northern regions of P. R. China (Table 1). The seeds were separated from mature berries, rinsed with water, and dried at room temperature on filter papers. The seeds were then kept in sealed tubes at 5 ± 1 C until use.
Table 1. Sampling information of six Solanum nigrum populations.

a Mean temperature during seed maturation represents the average daily temperature during maturation of S. nigrum seeds from July to October.
b Data from wheatA software, v. 1.3.7 (WheatA Big Data, Ningbo, China).
c The climate type is based on Köppen-Geiger system (Kottek et al. Reference Kottek, Grieser, Beck, Rudolf and Rubel2006). Appropriate coordinates were downloaded from the World Maps of Köppen-Geiger Climate Classification (http://koeppen-geiger.vu-wien.ac.at/present.htm, accessed July 21, 2021).
Germination and Seed Vigor Test
The S. nigrum seeds were surface sterilized with a 2% sodium hypochlorite solution for 10 min, then thoroughly rinsed with sterile distilled water (SDW) and placed in petri dishes containing two layers of damp sterile filter paper. Each population had three replicates of 40 seeds per petri dish, which were placed into incubators (Ningbo Jiangnan Instrument Factory) for germination. Seeds were regularly moistened with SDW according to the water loss in the petri dishes during the incubation period. The temperature of the seven incubators was set at 10, 15, 20, 25, 30, 35, and 40 C; the photoperiod was 12-h light/12-h darkness; and the relative humidity was maintained at 50%. Seeds with a radicle length greater than 1 mm were recorded as germinated, and percent germination (i.e., the number of seeds exhibiting radicle emergence) was monitored daily for 10 d; once seeds no longer germinated for three consecutive days, the experiment was ended (Liu et al. Reference Liu, Liu, Ye, Zhu, Chen, Jia, Xia, Shi, Jia and Zhang2015; Mohamed et al. Reference Mohamed, Kasem, Gobouri, Elkelish and Azab2020). At the end of the germination test, the ungerminated seeds were tested for viability with tetrazolium (2,3,5-tryphenyl tetrazolium chloride), and seeds with pink- or red-stained embryos after 3 h were considered viable for the estimation of germination percentage (Zhang et al. Reference Zhang, Wu, Zhang, Deng, Duan, Teixeira da Silva, Huang and Zeng2015).
Determination of Germination Parameters
Seed germination in response to temperature can be summarized by the cardinal temperatures (base, optimum, and ceiling temperatures), because temperature is a continuous variable that can be accurately measured and quantitatively analyzed. The optimal germination temperature of the S. nigrum seeds (the most rapid and highest germination for each population) was determined according to the germinability, germination potential, and germination index in seven incubators maintained at constant temperatures between 10 and 40 C at 5 C intervals. Then, the germination temperature of seeds was divided into two temperature ranges: suboptimal temperature and supraoptimal temperature. Under suboptimal temperature conditions (T < T 0), the model can be expressed as:
where θT(g) is the accumulated heat units above the base temperature (in degree-days), T is the germination temperature, T b is the base germination temperature, and t g is the days required to reach a cumulative germinability of g. At the same time, to predict the base germination temperature, we applied nonlinear logistic regression models according to Brown and Mayer (Reference Brown and Mayer1988) to fit for cumulative germinability g and germination time t:
where m, k, and b are empirically derived constants, m is approximately equal to the germinability, k is the growth rate of the germinability, and b is the parameter related to the germination delay. In this equation, the values of four parameters m, k, b, and T b are obtained through nonlinear regression, and the maximum fit between simulated and experimentally obtained data was achieved by an iterative technique using a quasi-Newton algorithm (Arana et al. Reference Arana, Gonzalez-Polo, Martinez-Meier, Gallo, Benech-Arnold, Sánchez and Batlla2016).
Thus, combining Equations 1 and 2, the accumulated GDD θT(g) can be calculated by the following equation:
Estimation of Three Fundamental Temperature Points
Based on the predictions of base temperature, the optimum temperature (T o) and ceiling temperature (T c) for germination were estimated according to Covell et al. (1986). Under suboptimal temperature conditions (T < T o), the speed of germination (1/t g , reciprocal of the time required for the germinability to reach a certain percentage g) is positively correlated with the germination temperature, and at supraoptimal temperatures (T > T o), it is negatively correlated. Therefore:
where θ1T (g) and θ2T (g) denote the accumulated GDD required for seed germination under suboptimal temperatures and supraoptimal temperatures, respectively. According to the process of seed germination under different temperature conditions, the time (t g ) required to obtain a germinability of 10% to 80% at 10% intervals was calculated based on statistical data. Linear regression equations of the seed germinability (1/t g , germinability g = 10%, 20%, 30%, 40%, 50%, 60%, 70%, and 80%) and germination temperature were established under suboptimal temperatures or supraoptimal temperatures. The intersection of the regression line and the x axis was the lower threshold of the germination temperature, and the intersection of the two regression lines was the optimal temperature for seed germination.
Data Analysis
The calculations of the germinability, germination potential (Wei et al. Reference Wei, Liu, Li, Zhao, Liu, Yu, Shen, Zhou, Zhu, Shu and Ma2020), and germination index (GI) (Chen et al. Reference Chen, Chen, Kong, Xia, Yan, Zhu and Mao2016; Hayat et al. Reference Hayat, Ahmad, Nasir, Khan, Ali, Hayat, Khan, Khan, Ma and Cheng2020; Zhou et al. Reference Zhou, Zhou, Zhang, Zhuang, Yang, Bazaka and Ostrikov2016) were as follows:
where G a is the number of all germinated seeds at the end of the germination period and G n is the total number of seeds tested.
where N t is the number of seeds germinated on day t, and D t represents the corresponding day of germination.
The data were analyzed using SPSS software v. 19.0 (IBM, Armonk, NY, USA). One-way ANOVA was carried out, followed by Duncan’s multiple range tests (P < 0.05).
Results and Discussion
Effect of Temperature on the Germination of Solanum nigrum
There were significant differences in the germinability of S. nigrum populations within the range of 10 to 40 C, as shown in Figures 1 and 2. Population XJ1600 had a wide temperature range of 15 to 30 C with high seed germination (>90%), with maximum germination (99.2%) occurring at 30 C. The optimum temperature for germination varied among populations. The differences in the germination of population JL1697 were not significant at constant temperatures from 15 to 30 C, but the highest germinability (79.2%) was at 20 C, and the optimum temperatures for the germination of populations NMG1704 and HLJ2134 were 25 and 30 C, respectively. The HN2160 and LN2209 populations showed a similar germination trend, in that they both germinated better at lower temperatures ranging from 15 to 20 C; the germinability for both at 15 C were 100% and 97.5%, respectively. Throughout the germination test, no seed germination was observed for the six tested populations of S. nigrum at 10 C and 40 C. This means that 40 C exceeds the maximum germination temperature, and 10 C is below the minimum temperature that can lead to successful S. nigrum seed germination. As shown in Figure 2, the germination potential displayed a similar tendency to the germinability. The germination potential of population XJ1600 was significantly higher than that of the other populations at the corresponding temperatures ranging from 15 to 35 C. Seeds of population XJ1600 began to germinate on the second day at 30 C, and germination peaked on the third day, with a germination potential of 87.5%. The changes in the germination potential showed that population XJ1600 had the most rapid germination, while that of population LN2209 was relatively slow in comparison with other populations.

Figure 1. Germination of Solanum nigrum seeds at 30 and 15 C with time.
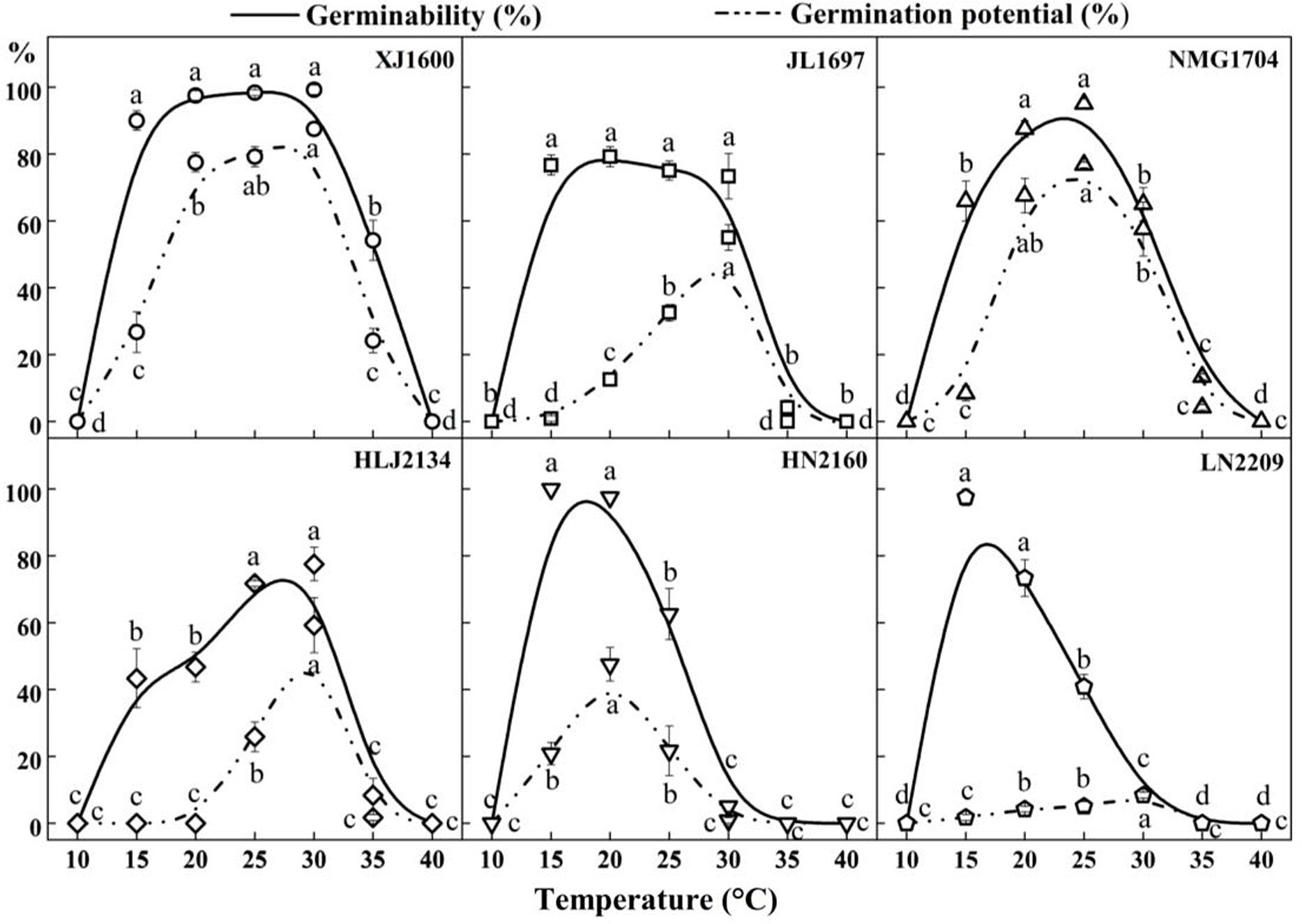
Figure 2. Effect of temperature on the seed germinability and germination potential of six Solanum nigrum populations (XJ1600, JL1697, NMG1704, HLJ2134, HN2160, and LN2209). The solid line and dotted lines correspond to the germinability and germination potential at 10, 15, 20, 25, 30, 35, and 40 C, respectively. Common lowercase letters indicate no significant difference (P < 0.05).
The GI can provide a sensitive indicator of seed vigor, with larger values suggesting that the seeds are more vigorous. As shown in Figure 3, in the temperature range of 15 to 35 C, the GI of population XJ1600 was obviously higher than that of the other populations at the corresponding temperatures. The variation in the GI of S. nigrum seeds from the six populations tested revealed that population XJ1600 was the most vigorous. Populations JL1697 and HLJ2134 attained maximum GIs of 7.6 and 7.7 at 30 C, respectively. When the temperature exceeded 30 C, the GI was significantly reduced. The population LN2209 had a maximum GI of only 6.2 at 15 C, indicating that seed vigor was relatively low.

Figure 3. Effect of temperature on the seed germination index of six Solanum nigrum populations (XJ1600, JL1697, NMG1704, HLJ2134, HN2160, and LN2209). Common lowercase letters indicate no significant difference (P < 0.05).
Our results suggest that increasing temperature significantly improved the germinability of S. nigrum seeds, shortened the germination time, and accelerated the germination process, similar to results for other species (Ooi et al. Reference Ooi, Auld and Denham2009). However, as the temperature increased, dormancy and mortality rates increased, while the germinability of S. nigrum seeds was significantly reduced at 35 C, and no seeds germinated at 40 C. It is possible that the high mortality rate under high temperature may place selective evolutionary pressure on the seed germination process. This pressure may select for reduced germination if a further increase in the temperature will lead to seed mortality, and this may also be an intrinsic response of seeds to exhibit high germinability at relatively high environmental temperatures within the normal range encountered at the site. Nevertheless, there were two populations in this study that germinated better at relatively low temperatures, and we speculate that the main reason for this result is that these two populations, which are located at more southerly latitudes than the other four populations, are found where there is adequate precipitation and sufficient conditions suitable for seed growth without relatively drastic temperature fluctuations; thus, the seeds began to germinate rapidly when temperature conditions reached a certain threshold.
Differences in the Base Temperature and Accumulated Temperature for Seed Germination
The base germination temperature is a fundamental biological parameter for the estimation of the accumulated GDD for seed germination, which is an expression of the adaptation of seed germination to its growth environment (Galíndez et al. Reference Galíndez, Seal, Daws, Lindow, Ortega-Baes and Pritchard2017). Germination can only occur when the ambient temperature exceeds the base temperature. Moreover, as an important driver of plant communities, habitat conditions intensely affect the expression of traits in offspring (Geshnizjani et al. Reference Geshnizjani, Snoek, Willems, Rienstra, Nijveen, Hilhorst and Ligterink2020). In the case of S. nigrum seeds, the destiny of the offspring is highly correlated with the population’s habitat at the time of seed development and maturity (Cendán et al. Reference Cendán, Sampedro and Zas2013; Figueroa et al. Reference Figueroa, Herms, Cardina and Doohan2010; Mohamed et al. Reference Mohamed, Kasem, Gobouri, Elkelish and Azab2020; Tielborger and Petru Reference Figueroa, Herms, Cardina and Doohan2010; Wijewardana et al. Reference Wijewardana, Reddy, Krutz, Gao and Bellaloui2019). Based on the optimum temperatures for germination of the six populations initially obtained from germination tests, the parameter estimations, base temperature and cumulative temperature for germination, were calculated by using a nonlinear fit of the thermal time model and logistic functions under suboptimal temperatures for each of the six populations. The values of the parameters are shown in Table 2. The base germination temperature varied among the six populations from different regions, with the lowest base temperature observed in population JL1697 (2.3 C) and the highest observed in population NMG1704 (6.4 C). The lowest value of the cumulative temperature required to reach up to 50% germination occurred in population HN2160 (50.3 C·d), and the highest occurred in population HLJ2134 (106.0 C·d). In previous studies, the base temperature for the germination of temperate species ranged from 0 to 4 C, and the mean range of accumulated GDD required for the germination of temperate species was 15 to 94 C·d (Trudgill et al. Reference Trudgill, Squire and Thompson2000, Reference Trudgill, Honek, Li and Van Straalen2005). The lowest base temperature we estimated was 2.3 C, and the highest was 6.4 C. The base germination temperatures of four populations of S. nigrum were greater than 4 C, and the accumulated GDD of population HLJ2134 was 106.0 C·d, which was higher than 94 C·d, which was significantly different from the results of prior studies (Trudgill et al. Reference Trudgill, Squire and Thompson2000).
Table 2. The parameter values m, k, b, and T b obtained by nonlinear regression analysis and the calculated average accumulated growing degree days (GDD) θT (50%). a

a Data are presented as the mean ± SE (n = 3). The parameters have a fixed value and can be obtained by a nonlinear regression equation. The maximum fit between simulated and experimentally obtained data was achieved by an iterative technique using a quasi-Newton algorithm (Arana et al. Reference Arana, Gonzalez-Polo, Martinez-Meier, Gallo, Benech-Arnold, Sánchez and Batlla2016). m is an approximation of the germinability; k is the rate of growth of germination; b is a parameter related to germination delay; T b is the basal temperature of germination; θT (50%) is the accumulated GDD required when the germinability reaches 50%.
b R2 is the coefficient of determination.
c RMSE, root mean-square error of empirical and theoretical data fitting.
Figure 4 shows the logistic curve fits between the accumulated GDD required for seed germination and the cumulative germination at constant temperatures of 10, 15, 20, 25, and 30 C. The cumulative germination of the six populations tested first increased slowly with increasing accumulated GDD, then increased rapidly, and finally reached their maxima. Validity of the models can be assessed through goodness of the data fit, for which the coefficient of determination (R2) of the six populations was above 0.85, indicating that the models described the data well. We analyzed data obtained for the base temperature and accumulated GDD, as seen in Figure 5. The dotted line presents the linear regression between the base temperature and accumulated GDD for the six populations tested, and the solid line shows the linear relationship among the other five populations except HLJ2134. There was a significant negative correlation between the base temperature and accumulated GDD for seed germination (P < 0.05), which means that populations with a lower base temperature required a higher accumulated GDD to germinate. Trudgill et al. (Reference Trudgill, Honek, Li and Van Straalen2005) indicated that seed germination for populations adapted to colder temperatures required higher accumulated GDD and a lower T b compared with populations adapted to warmer environments. Population HLJ2134 was located in the most northerly location among the six populations, where the average annual temperature is the lowest, and seeds from this population required a higher accumulated GDD for seed germination. This result means that seeds could not germinate during brief periods of high temperature, reducing the risks endured in difficult climatic conditions and resisting undesirable climate changes. However, population HN2160 requires the lowest accumulated GDD for germination and had the most southerly location among the six populations; thus, it is reasonable to assume that areas with higher average annual temperatures require lower accumulated GDD and a shorter time for completing germination.

Figure 4. The fitted curve between accumulated growing degree days (GDD) and cumulative germination of six populations of Solanum nigrum based on data from the experiments at constant temperatures. The curve is plotted starting at the calculated suboptimal temperature of each population. The graphs for populations XJ1600 and JL1697 are drawn with data from five constant temperatures (10, 15, 20, 25, and 30 C); those for populations NMG1704 and HLJ2134 are drawn with data from four constant temperatures (10, 15, 20, and 25 C); and those for populations HN2160 and LN2209 are drawn with data from three constant temperatures (10, 15, and 20 C).

Figure 5. The linear relationship between the base temperature and accumulated growing degree days (GDD) for germination of five populations of Solanum nigrum was plotted. The dotted lines represent the fitted curves between the base temperature and accumulated GDD for the five populations, the degree of fit (R2) was 0.33; the solid lines represent the fitted curves between the base temperature and accumulated GDD for populations XJ1600, JL1697, NMG1704, HN2160, and LN2209; the degree of fit (R2) was 0.96.
Our results also verified that there is a negative correlation between the base temperature and the accumulated GDD of seed germination, as shown in previous studies. By fitting the base temperature and accumulated GDD for the six populations tested, we found that the base temperature could only explain 33.2% of the variation in accumulated GDD, but after removing population HLJ2134 and fitting the data a second time, the base temperature could explain 95.7% of the variation in accumulated GDD. These results indicate that seed habitat is not the only factor explaining variation in accumulated GDD, which is also influenced by other factors, such as the origin of the population, phylogenetic level, and maternal genetics (Trudgill et al. Reference Trudgill, Squire and Thompson2000).
Prediction of the Optimum Temperature and Ceiling Temperature for Germination
The accumulated GDD theory is based on the use of three cardinal temperature points, and it is believed that between the base temperatures and optimum temperatures, seed germination accelerates linearly with temperature. Our studies showed that the effect of temperature on seed germinability was actually nonlinear, and directly using linear models of effective accumulated GDD or active accumulated GDD to simulate the seed germination process may lead to biased predictions. Thus, we have proposed and successfully applied nonlinear thermal time models to describe seed germination.
A linear regression equation was fit to the germinability (1/t g ) and temperature of S. nigrum seeds from the six populations tested, as shown in Figure 6. The germinability was significantly positively correlated with temperature between the base temperature and optimum temperature and was negatively correlated with temperature between the optimum temperature and ceiling temperature. We estimated the optimum temperature and ceiling temperature for each of the six populations tested with a regression equation, and the results are presented in Table 3. The predicted optimum temperatures were compared with the optimum temperatures observed in previous germination tests, and the minimum error between the observed and predicted values was 0.3 C for population XJ1600, and the maximum error was 4.0 C for population LN2209. The predicted ceiling temperatures for the six populations ranged from 34.7 to 40.0 C. Notably, the ceiling temperatures predicted for populations HN2160 and LN2209 were 35.0 and 34.7 C, respectively, which were relatively lower than those of the other populations; the trend was the same as that for the optimum temperature for germination.

Figure 6. Linear fit between the seed germinability (1/t g ) and the constant germination temperature of six populations of Solanum nigrum.
Table 3. The optimum temperature (T o) and ceiling temperature (T c) for seed germination across six populations of Solanum nigrum estimated based on the regression analysis of germination temperature.

a “P” represents the predicted value obtained from the regression equation; “O” represents the data from previous observations.
b Linear regression equations of the seed germinability (1/t g , germinability g=10%, 20%, 30%, 40%, 50%, 60%, 70% and 80%) and germination temperature were established under suboptimal temperatures or supraoptimal temperatures. The intersection of the regression line and the x axis was the threshold of the germination temperature, and the intersection of the two regression lines was the optimal temperature for seed germination.
Temperature is a known determining factor in seed germination. The germination response of S. nigrum to temperature varied among populations. Interestingly, we found populations from more northerly latitudes had a higher and wider range of optimum temperatures for germination. The optimum temperature for germination of populations XJ1600, JL1697, and HLJ2134 was 30 C, and those for the populations NMG1704, HN2160, and LN2209 were 25, 20, and 15 C, respectively. Based on the nonlinear fit and thermal time models, the base temperatures and accumulated GDD for germination also differed among populations and showed a significant negative correlation. Our findings suggest that germination of S. nigrum populations is highly adapted to their environments. This adaption is possibly a response by which germinating seeds can avoid adverse growing conditions. Knowledge obtained in this study will be helpful in predicting seedling emergence and developing the timing for optimal control solutions for S. nigrum.
Acknowledgments
The authors are very grateful to the associate editor Chenxi Wu and anonymous reviewers for their valuable comments and suggestions, which helped improve the quality of this article. This work is funded by the Key Research and Development Program of Special Funds for Construction Corps (2018AA006) and the National Key Research and Development Program of China (2018YFD0200602). No conflicts of interest have been declared.












