Article contents
Heating rate effects in simulated liquid Al2O3
Published online by Cambridge University Press: 30 November 2005
Abstract
The heating rate effects in simulated liquid Al2O3 have been
investigated by Molecular Dynamics (MD) method. Simulations were done in the
basic cube under periodic boundary conditions containing 3000 ions with
Born-Mayer type pair potentials. The temperature of the system was
increasing linearly in time from the zero temperature as $T(t)=T_0 +\gamma
t$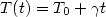 , where $\gamma $
, where $\gamma $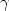 is the heating rate. The heating rate dependence of
density and enthalpy of the system was found. Calculations show that static
properties of the system such as the coordination number distributions and
bond-angle distributions slightly depend on $\gamma $
is the heating rate. The heating rate dependence of
density and enthalpy of the system was found. Calculations show that static
properties of the system such as the coordination number distributions and
bond-angle distributions slightly depend on $\gamma $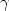 . Structure of
simulated amorphous Al2O3 model with the real density at the
ambient pressure is in good agreement with Lamparter's experimental data.
The heating rate dependence of dynamics of the system has been studied
through the diffusion constant, mean-squared atomic displacement and
comparison of partial radial distribution functions (PRDFs) for 10% most
mobile and immobile particles with the corresponding mean ones. Finally, the
evolution of diffusion constant of Al and O particles and structure of the
system upon heating for the smallest heating rate was studied and presented.
And we find that the temperature dependence of self-diffusion constant in
the high temperature region shows a crossover to one which can be described
well by a power law, $D\propto (T-T_c )^\gamma $
. Structure of
simulated amorphous Al2O3 model with the real density at the
ambient pressure is in good agreement with Lamparter's experimental data.
The heating rate dependence of dynamics of the system has been studied
through the diffusion constant, mean-squared atomic displacement and
comparison of partial radial distribution functions (PRDFs) for 10% most
mobile and immobile particles with the corresponding mean ones. Finally, the
evolution of diffusion constant of Al and O particles and structure of the
system upon heating for the smallest heating rate was studied and presented.
And we find that the temperature dependence of self-diffusion constant in
the high temperature region shows a crossover to one which can be described
well by a power law, $D\propto (T-T_c )^\gamma $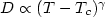 . The critical temperature
Tc is about 3500 K and the exponent $\gamma $
. The critical temperature
Tc is about 3500 K and the exponent $\gamma $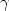 is close to 0.941 for Al
and to 0.925 for O particles. The glass phase transition temperature
Tg for the Al2O3 system is at anywhere around 2000 K.
is close to 0.941 for Al
and to 0.925 for O particles. The glass phase transition temperature
Tg for the Al2O3 system is at anywhere around 2000 K.
- Type
- Research Article
- Information
- Copyright
- © EDP Sciences, 2006
References
- 5
- Cited by




