Overall, 50-75% of patients with variously defined fatigue syndromes have comorbid mood or anxiety disorders (Reference Katon, Walker, Bock and WhelanKaton & Walker, 1993; Reference WesselyWessely, 1995), leading some investigators to suggest that chronic fatigue is simply a forme fruste of anxiety or depressive states (Reference Manu, Lane and MatthewsManu et al, 1988). However, these subjects' failure to respond to antidepressant pharmacotherapy (Reference Vercoulen, Swanink and ZitmanVercoulen et al, 1996) and evidence of neuroendocrine abnormalities that diverge from those in patients with depression (Reference DemitrackDemitrack, 1997) are more consistent with chronic fatigue being a distinct disorder. The nature of the association between these two disorders is unclear. Although two prospective studies of primary care cohorts failed to find evidence (over 12 months and 18 months respectively) that pure fatigue without concurrent depression was a causal risk factor for pure depression or vice versa (Reference Hickie, Koschera and Hadzi-PavlovicHickie et al, 1999a ; Reference van der Linden, Chalder and Hickievan der Lindenet al, 1999), one large twin study identified three different genetic factors, one of which was unique for fatigue, accounting for 44% of its total genetic variance, while the other two contributed to symptoms of both fatigue and anxiety/depression (Reference Hickie, Kirk and MartinHickie et al, 1999b ). Unfortunately, none of these studies focused on chronic debilitating fatigue, and twins had a minimum age of 50 years and a mean age of 62 years so that adjusting for age-correlated medical conditions was problematic. The purpose of the study reported here was to conduct a co-twin control analysis of psychological distress in female twin pairs discordant for varying degrees of chronic fatigue. The co-twin control method is a powerful design for controlling confounds due to genetic factors and shared environment and can determine whether the source of the relationship between two traits is due to common genetic factors (i.e. genetic covariation; Reference Kendler, Karkowski and PrescottKendler et al, 1999).
METHOD
Identification of twins and data collection
Twins were recruited through a variety of sources, including advertisements in patient support group newsletters (55%); through clinicians and researchers familiar with chronic fatigue syndrome (CFS) (10%); solicitations placed on CFS electronic bulletin boards (18%); twin researchers or organisations (6%); friends or relatives (3%); and through various other means (9%). All recruitment efforts emphasised that fatigued twins were desired regardless of either the health of the co-twin or a definitive diagnosis of CFS. Written, informed consent was obtained from each participant in accordance with the regulations of our institutional Human Subjects Office.
A detailed account of the construction of the registry has been presented elsewhere (Reference Buchwald, Herrell and AshtonBuchwald et al, 1999). Briefly, all study participants completed an extensive mailed survey questionnaire that included questions on fatigue and the CFS symptom criteria according to the 1994 revised Centers for Disease Control and Prevention (CDC) working case definition (Reference Fukuda, Scourfield and MartinFukuda et al, 1994). For non-fatigued twins, a control version of questions was used that did not refer to fatigue. In addition, participants were asked about their demographic characteristics and, to help determine zygosity, questions on similarity as children. A trained research assistant administered the Diagnostic Interview Schedule (DIS) version III—A (Reference Robins and HelzerRobins & Helzer, 1985) by telephone to all twin pairs of whom at least one twin reported fatigue of at least 6 months in duration. The DIS assigns current and life-time diagnoses using a computer algorithm based on the criteria of the DSM—III—R (American Psychiatric Association, 1987). The interview was used to assess the CDC-defined psychiatric diagnoses exclusionary to CFS.
To determine health conditions, the questionnaire contained a checklist of selfreported medical problems; twins indicated whether a condition was currently active or had resolved, and whether a physician had evaluated the condition. The application of the CDC-defined exclusionary medical conditions to the checklist was determined by consensus of two general internists, a psychiatrist with expertise in CFS, an infectious disease specialist, and an internist/ emergency room physician with knowledge of, but little exposure to, patients with CFS. Examples from the comprehensive list of exclusionary disorders included (but were not limited to) steroid-dependent asthma, infectious hepatitis, diabetes, cancer (other than skin), congestive heart failure, stroke, cirrhosis, multiple sclerosis and systemic lupus erythematosus. To assess the performance of our list of exclusionary conditions, subjects' self-reported health conditions were compared with physician confirmation of these diagnoses by chart review and telephone contact with treating physicians for a subsample of twins. Among 44 twins, we did not find any fatigued participant to be ineligible because of an exclusionary condition that was missing or inaccurately reported on the checklist. Conversely, no exclusionary conditions were observed in any twin who self-reported good health.
Definitions of fatigue
We used three progressively more stringent (but not mutually exclusive) case definitions of chronic fatigue. The first definition, chronic fatigue, was based on the response to the single question: ‘Have you been fatigued for at least 6 months?’ No further inclusionary or exclusionary conditions were applied.
The second definition, chronic fatigue not explained by medical exclusions, classified all twins according to the CDC CFS research definition using data obtained solely from the mailed questionnaire. An algorithm was developed that defined chronic fatigue using both the inclusionary and medical exclusionary components of the CDC case definition (Reference Fukuda, Scourfield and MartinFukuda et al, 1994). The exclusionary medical criteria were applied to both the chronically fatigued and non-fatigued twins. To be classified as having medically unexplained chronic fatigue, twins were required to report fatigue of at least 6 months' duration that was not lifelong and that resulted in a substantial reduction of occupational, educational, social or personal activities. Furthermore, subjects had to endorse the presence of four or more of eight CDC CFS symptom criteria: impaired memory or concentration, sore throat, tender glands, aching or stiff muscles, multi-joint pain, new headaches, unrefreshing sleep and post-exertional fatigue (Reference Fukuda, Scourfield and MartinFukudaet al, 1994). Twins were excluded from this definition of chronic fatigue if they had a body mass index of 45 kg/m2 or more (as stipulated in the CDC criteria) or reported any of the exclusionary medical conditions.
The third definition, chronic fatigue not explained by medical or psychiatric exclusions, further restricted our sample on the basis of the DIS-generated psychiatric diagnoses considered exclusionary by the CDC case definition. These included life-time mania, hypomania, bipolar disorder, schizophrenia, major depression with psychotic or melancholic features, anorexia or bulimia nervosa, and current alcohol or substance abuse or dependence. Identical psychiatric exclusionary criteria were used with both fatigued and nonfatigued twins.
Determination of zygosity
Studies have shown that questions about childhood similarity in twin pairs can be used to classify zygosity with an accuracy of 95-98% compared with biological indicators (Reference TorgersenTorgersen, 1979; Reference Eisen, Neuman and GoldbergEisen et al, 1989). Twin pairs in which both members answered affirmatively to multiple questions about similarity in the mailed questionnaire (e.g. ‘When you were young, were you as alike as peas in a pod?’) were classified as monozygotic (MZ), and those pairs in which both twins denied this degree of similarity were considered to be dizygotic (DZ).
General Health Questionnaire
The General Health Questionnaire (GHQ) is a 28-item self-report instrument that measures psychological and somatic distress in the ‘past few weeks’ on a four-point scale (Reference Goldberg and HillierGoldberg & Hillier, 1979). The questionnaire has been recommended by the National Institutes of Health and the National Institutes of Health and the National Institute of Mental Health for use in CFS research (Reference Schluederberg, Straus and PetersonSchluederberg et al, 1992). The GHQ contains four sub-scales: somatic symptoms; anxiety and insomnia; social dysfunction; and severe depression. The sub-scales are scored by summing the values for endorsed items (higher scores suggest greater distress). In addition, a total score cut-off can be used to determine the sensitivity and specificity of the GHQ in various populations (e.g. Reference Buchwald, Pearlman and KithBuchwald et al, 1997).
Statistical analysis
The initial descriptive analysis examined the mean levels of each of the four GHQ scale scores according to the three definitions of chronic fatigue. Formal statistical analysis compared the intra-pair mean differences for each GHQ scale score using paired t-tests. Examination of potential genetic covariation between chronic fatigue and the GHQ scale scores compared the magnitude of GHQ score differences between the fatigued and nonfatigued twins within MZ and DZ pairs. This approach to analysis was based on the methods articulated by Kendler et al (Reference Kendler, Karkowski and Prescott1999).
By comparing the size of the MZ and DZ intra-pair mean differences in GHQ scores using unmatched t-tests, it is possible to gain insight into the relative magnitude of genetic and environmental influence on both chronic fatigue and psychological distress. For example, if the intra-pair difference for the GHQ severe depression score in MZ twins were zero and a large difference were noted in DZ twins, this would strongly suggest that the association between chronic fatigue and severe depression as measured by GHQ is at least partly due to a shared genetic influence. Conversely, if the effects in MZ and DZ pairs were approximately comparable, this would suggest that the phenotypic association is not due to a common genetic influence on both traits.
RESULTS
Of 233 twin pairs identified, complete data were available for both members of 204 pairs (88%). Among these, 169 pairs were female—female, 13 were male—male, and 22 were male—female twins. This study was restricted to the 100 fatigue-discordant female—female twins since the samples of male—male and male—female twins were too small to analyse. Table 1 presents the characteristics of the female—female twins in each of the progressively more stringent chronic fatigue samples.
Table 1 Zygosity and demographic characteristics of female—female chronic fatigue discordant twins by stringency of fatigue classification

| Characteristic | Chronic fatigue ≥ 6 months | Chronic fatigue not due to medical exclusions | Chronic fatigue not due to medical or psychiatric exclusions |
|---|---|---|---|
| Zygosity (n) | |||
| Monozygotic | 69 | 54 | 43 |
| Dizygotic | 31 | 24 | 18 |
| Demographics | |||
| White (%) | 100 | 100 | 100 |
| Date of birth: year (s.e.) | 1953 (1.1) | 1953 (1.3) | 1954 (1.4) |
| Education (years): mean (s.e.) | 14.4 (0.2) | 14.6 (0.2) | 14.6 (0.2) |
| Married (%) | 62 | 60 | 65 |
| Fatigue1 | |||
| Duration (years): mean (s.e.) | 6.2 (0.8) | 5.9 (0.8) | 6.6 (1.0) |
| Severity: mean (s.e.)2 | 7.7 (0.2) | 7.6 (0.2) | 7.7 (0.3) |
The zygosity distribution of twins in all three samples was similar, with a preponderance of MZ twin pairs. Fatiguediscordant twin pairs were not different on any of the demographic characteristics at all three levels of analysis. All of the twins were White; on average, the twins were in their mid-40s, had approximately 15 years of education, and the majority were married. The fatigued twins had experienced fatigue for about 6 years with a mean severity of more than 7.5 on a 1 to 10 scale (1, none; 10, a great deal).
The means and associated 95% confidence intervals for each of the GHQ sub-scales are presented separately for MZ and DZ pairs and according to each of the three definitions of chronic fatigue in Figs 1,2,3,4.
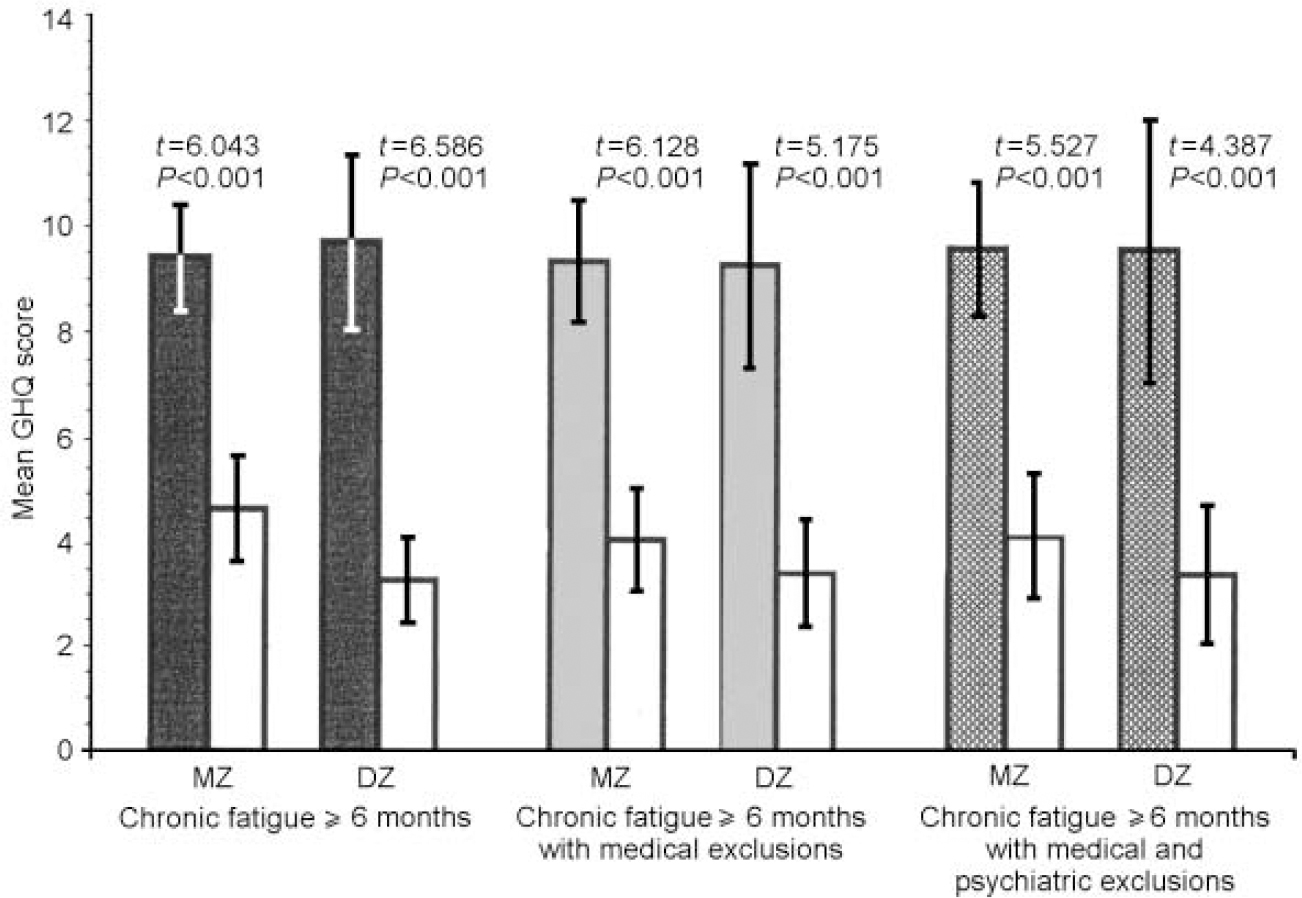
Fig. 1 Mean values of General Health Questionnaire (GHQ) somatic symptoms scores for monozygotic (MZ) and dizygotic (DZ) twins by stringency of fatigue classification, in comparison with non-fatigued co-twins (white bars).
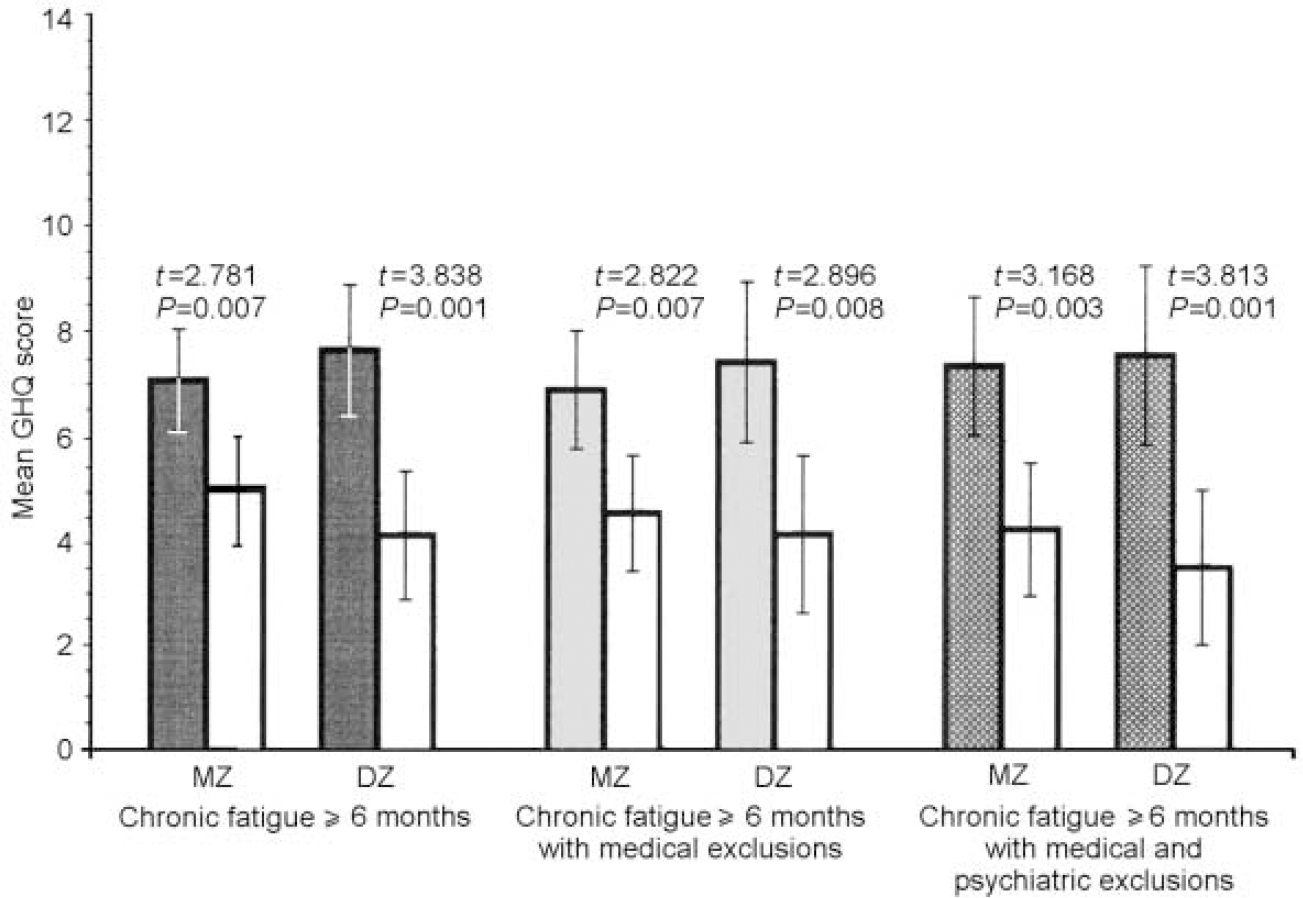
Fig. 2 Mean values of General Health Questionnaire (GHQ) anxiety and insomnia scores for monozygotic (MZ) and dizygotic (DZ) twins by stringency of fatigue classification, in comparison with non-fatigued co-twins (white bars).
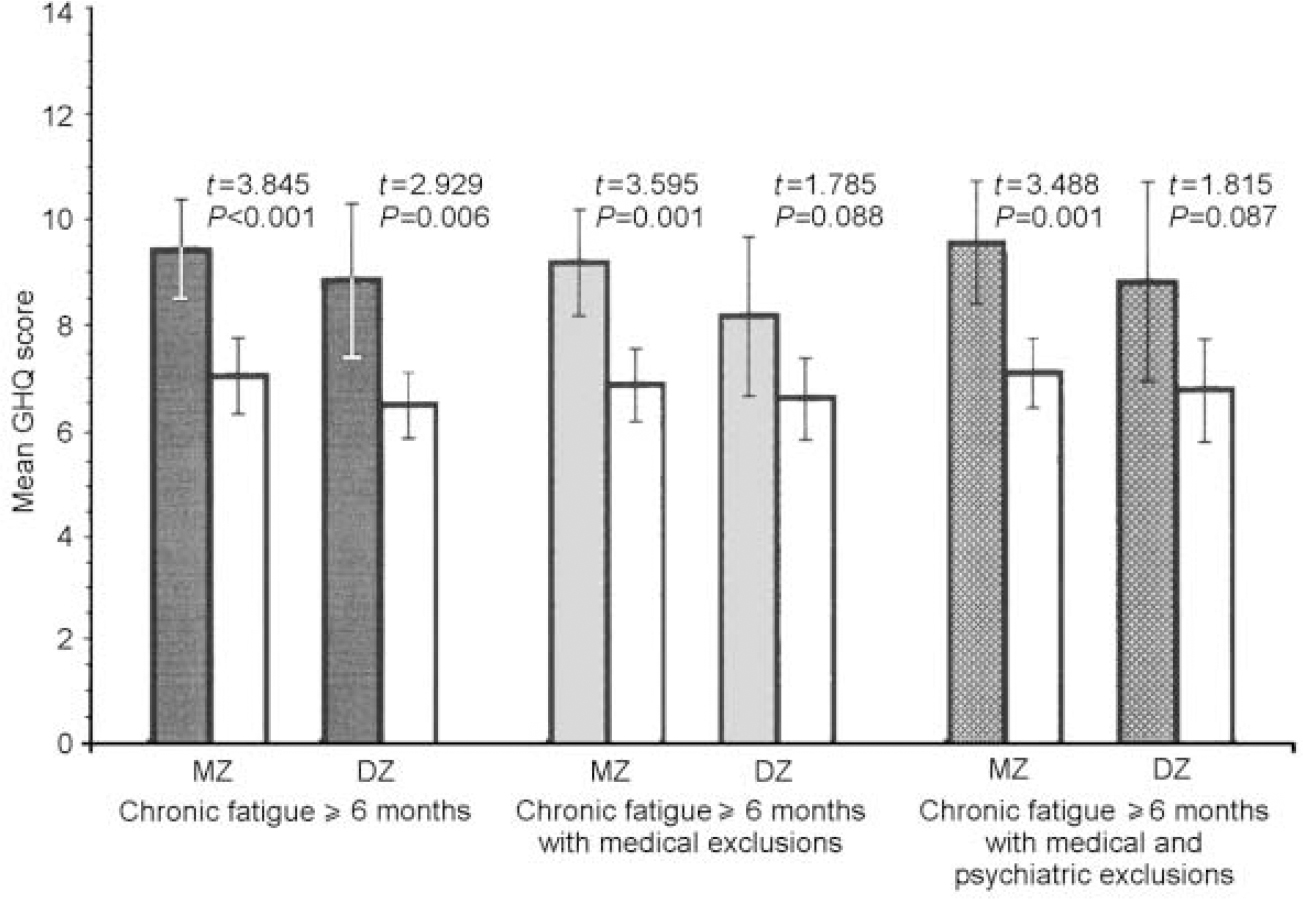
Fig. 3 Mean value of General Health Questionnaire (GHQ) social dysfunction scores for monozygotic (MZ) and dizygotic (DZ) twins by stringency of fatigue classification, in comparison with non-fatigued co-twins (white bars).
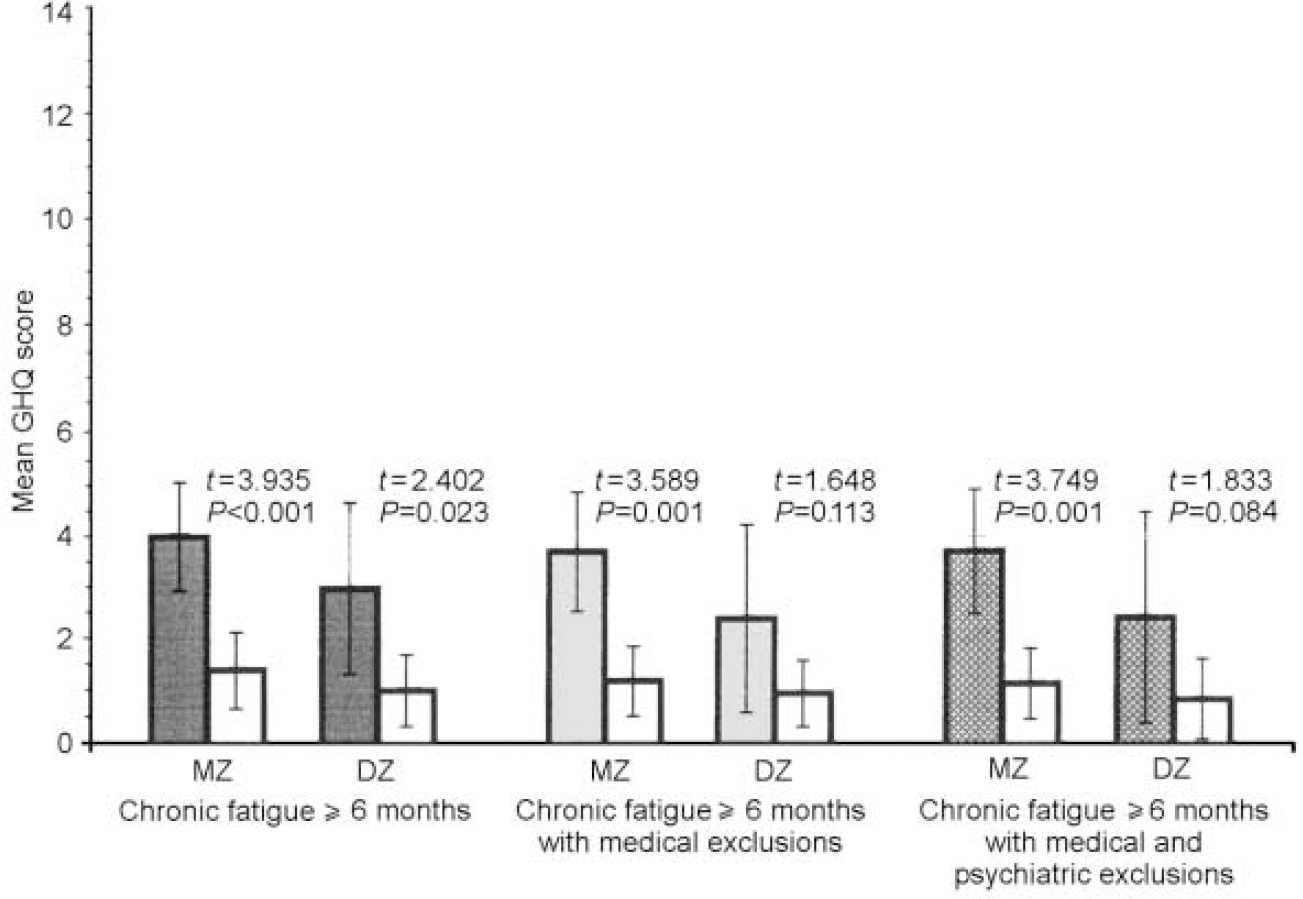
Fig. 4. Mean values of General Health Questionnaire (GHQ) severe depression scores for monozygotic (MZ) and dizygotic (DZ) twins by stringency of fatigue classification, in comparison with non-fatigued co-twins (white bars).
The data were similar across zygosity, sub-scale score and fatigue definition. In both MZ and DZ twin pairs, regardless of definition, the fatigued twins were more somatically preoccupied and anxious than the non-fatigued twins (all P values ≤0.01). Fatigued MZ twins at all three levels of fatigue definition were more socially dysfunctional and depressed than their non-fatigued counterparts (all P values ≤ 0.001), whereas these differences were significant for DZ twins only in the sample with the first and broadest definition of chronic fatigue (social dysfunction: t=2.929, P=0.006; severe depression:t=2.402, P=0.023). Although not significant, fatigued DZ twins with the two more stringent definitions of fatigue were also more socially dysfunctional and depressed than their non-fatigued counterparts.
Table 2 presents the comparisons of MZ and DZ twin pairs on the intra-pair mean differences for each GHQ sub-scale using the three increasingly stringent definitions of fatigue. None of the intra-pair GHQ scale differences was significantly different between the MZ and DZ twin pairs. However, larger intra-pair differences for the somatic symptoms and the anxiety and insomnia sub-scales were consistently noted in DZ twins. In contrast, for social dysfunction and depression, MZ intra-pair differences tended to be larger than DZ intra-pair differences.
Table 2 The General Health Questionnaire sub-scale intra-pair mean differences for female—female monozygotic (MZ) and dizygotic (DZ) twin pairs by stringency of fatigue classification
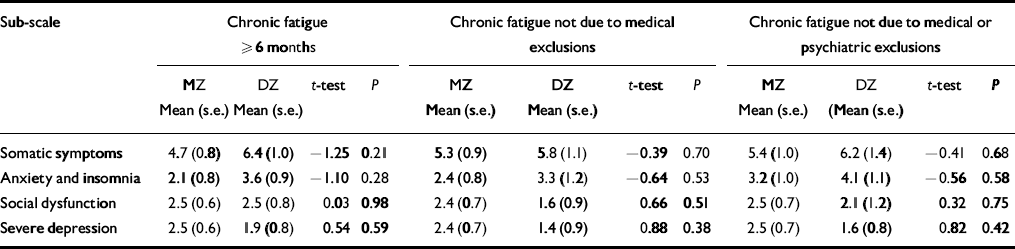
| Sub-scale | Chronic fatigue ≥ 6 months | Chronic fatigue not due to medical exclusions | Chronic fatigue not due to medical or psychiatric exclusions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ Mean (s.e.) | DZ Mean (s.e.) | t-test | P | MZ Mean (s.e.) | DZ Mean (s.e.) | t-test | P | MZ Mean (s.e.) | DZ (Mean (s.e.) | t-test | P | |
| Somatic symptoms | 4.7 (0.8) | 6.4 (1.0) | -1.25 | 0.21 | 5.3 (0.9) | 5.8 (1.1) | -0.39 | 0.70 | 5.4 (1.0) | 6.2 (1.4) | -0.41 | 0.68 |
| Anxiety and insomnia | 2.1 (0.8) | 3.6 (0.9) | -1.10 | 0.28 | 2.4 (0.8) | 3.3 (1.2) | -0.64 | 0.53 | 3.2 (1.0) | 4.1 (1.1) | -0.56 | 0.58 |
| Social dysfunction | 2.5 (0.6) | 2.5 (0.8) | 0.03 | 0.98 | 2.4 (0.7) | 1.6 (0.9) | 0.66 | 0.51 | 2.5 (0.7) | 2.1 (1.2) | 0.32 | 0.75 |
| Severe depression | 2.5 (0.6) | 1.9 (0.8) | 0.54 | 0.59 | 2.4 (0.7) | 1.4 (0.9) | 0.88 | 0.38 | 2.5 (0.7) | 1.6 (0.8) | 0.82 | 0.42 |
DISCUSSION
This study indicates that twins suffering from chronic fatigue have increased symptoms of psychological distress in comparison with their non-fatigued counterparts. Regardless of zygosity, chronic fatigue was associated with high scores in each of the four GHQ domains. This pattern was consistent across the three levels of fatigue stringency, suggesting that prolonged fatigue is the critical symptom in the association between chronic fatigue-related disorders and psychological distress. The lack of statistical significance in a few of the DZ comparisons is more likely to be a result of reduced power (small sample sizes) than differences in the impact of fatigue definition on psychological distress in DZ twins, especially since the level of significance approached 0.10 in all instances. These findings confirm other studies documenting a strong association between symptoms of fatigue and both psychological distress and psychiatric disorder (Reference Manu, Matthews and LaneManu et al, 1989; Reference Katon, Walker, Bock and WhelanKaton & Walker, 1993; Reference Wessely, Chalder and HirschWessely et al, 1996). Although the existence of this association and its presence in a substantial majority of fatigue cases is now well accepted, the nature of this association (does fatigue cause psychological distress or vice versa, or are they both manifestations of a common third factor?) is still in dispute.
Nature of the fatigue—distress association
The co-twin control study provides a unique approach to exploring the nature of the association between chronic fatigue and psychological distress. The design controls for the potential confounding influence of genetic and early environmental factors in a way that is not possible using standard research designs. Our results are clear regarding the absence of a common genetic factor for chronic fatigue and various domains of psychological distress, and consistent with evidence from a prospective primary care study that fatigue causes depression (odds ratio of 1.4; Reference Hickie, Koschera and Hadzi-PavlovicHickie et al, 1999a ). This finding is at variance with results from a previous study that found two genetic factors common to fatigue and distress (Reference Hickie, Kirk and MartinHickie et al, 1999b ), suggesting that the association between fatigue and distress is more likely to be environmental in origin. However, it is possible that our small sample size accounted for our failure to find genetic covariation between fatigue and measures of psychological distress. We also used a less age-restricted twin sample, and more refined and multiple definitions of fatigue, which may account for some of the disparities in results.
Association of fatigue with anxiety v. depression
Although our results did not demonstrate significant genetic covariation, there was an interesting pattern of differences across the four GHQ sub-scales. For somatic and anxiety symptoms, the larger intra-pair differences in the DZ twins suggest that some portion of these measures may be due to genetic covariation that was not detected owing to small sample size. Conversely, for severe depression, the MZ intra-pair difference was larger than that observed for DZ pairs (regardless of the stringency of the chronic fatigue definition), which argues against a common genetic influence. This is consistent with previous speculations that chronic fatigue most closely resembles ‘atypical depression’ with its prominent anxiety and somatic symptoms, tendency to cortisol hyposecretion, and poor response to conventional antidepressant pharmacotherapy (Reference Gold, Licinio and WongGold et al, 1995; Reference Terman, Levine and TermanTerman et al, 1998).
The evidence for a genetic influence on depression is abundant from numerous twin and family studies (Reference Moldin, Reich and RiceMoldinet al, 1991; Reference Kendler, Neale and KesslerKendleret al, 1993; Reference Lyons, Eisen and GoldbergLyonset al, 1998). However, the role of genetic effects in fatigue, more specifically chronic fatigue, is less well documented and has only recently emerged. Studying Australian twins, researchers demonstrated a significant heritability (44%) for a continuous measure of fatigue (Reference Hickie, Kirk and MartinHickie et al, 1999b ). Importantly, a component of both unique and shared (with depression) genetic influence on chronic fatigue was observed. Other investigators also have described a significant genetic influence on fatigue (54%) in a British sample of young twins (Reference Farmer, Scourfield and MartinFarmer et al, 1999). However, neither the Australian nor the British studies attempted to define a discrete illness phenotype of chronic fatigue. Using the twins from the registry described here, we found a significant heritable influence on a CFS-like illness (51%), defined according to the CDC 1994 criteria (Reference Buchwald, Herrell and HartmanBuchwald et al, 2001).
Limitations
This study has several limitations. First, the method used to identify the CFS twins was not ideal. Solicitation by advertisement resulted in a volunteer sample of twin pairs with the potential for ascertainment biases. However, the more desirable strategy of systematically identifying twins with chronic fatigue and CFS from a well-defined, truly population-based twin registry is not readily accomplished in the USA. Likewise, the selected nature of the sample, which had more female-female twin pairs and a long duration of fatigue, may have increased the probability of comorbid psychiatric illness, therefore inflating GHQ score differences between fatigued and non-fatigued twins. However, the rates of mood and anxiety disorders among our twins were comparable to those observed in clinical samples (Reference Katon, Walker, Bock and WhelanKaton & Walker, 1993). Second, the number of DZ twins and the overall number of twins discordant for chronic fatigue that were identified were not large. Finally, we relied on self-report to define and measure fatigue and exclusionary medical conditions. However, we closely followed the CFS symptom and psychiatric criteria as articulated by CDC. In addition, our three definitions of fatigue were consistent with those used in several recent epidemiological studies (Reference Nisenbaum, Reyes and MawleNisenbaum et al, 1998; Reference Steele, Dobbins and FukudaSteele et al, 1998; Reference Jason, Richman and RademakerJason et al, 1999).
Despite these shortcomings, this study makes several contributions to the field. Using three discrete definitions of fatigue, we have provided evidence that prolonged fatigue is the critical factor in the association of psychological distress with chronic fatigue. In addition, the absence of a common genetic factor for fatigue and various domains of psychological distress suggests that the relationship between fatigue and distress is largely, if not exclusively, environmental, although the direction of effect is unknown. The contribution of overlapping definitions to this relationship is unknown, but must also be acknowledged. If there is any small genetic relationship between fatigue and distress, the pattern of differences between various measures of distress and fatigue suggests this is most likely to involve anxiety and somatisation rather than depression, suggesting a relationship between fatigue and ‘atypical’ (i.e. anxious) forms of depression.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Prolonged fatigue, regardless of definition, is the critical symptom accounting for the clear association between chronic fatigue syndrome (CFS) and psychological distress.
-
• The relationship between CFS and psychological distress appears to be largely environmental in origin, although the direction of causality is unclear.
-
• Chronic fatigue may more closely resemble atypical, anxious forms of depression, rather than classic depression.
LIMITATIONS
-
• The small sample size provided only limited power to detect genetic covariation.
-
• The twin sample was not population-based but highly selected, which could have biased results.
-
• Reliance on self-reports for all measures of psychological distress and fatigue could also bias results, although the validity of these measures is fairly solid.
Acknowledgement
This research is supported by grant U19 A138429 from the National Institutes of Health (D. B.).











eLetters
No eLetters have been published for this article.