Appropriate nutrition during pregnancy and lactation is essential, given increased nutritional requirements during these periods, to promote both maternal and newborn growth and well-being. In resource-poor settings, pregnant women are often exposed to a low dietary diversity (or quality), which contributes to inadequate macronutrient and micronutrient intakes( Reference Haileslassie, Mulugeta and Girma 1 – Reference Lee, Talegawkar and Merialdi 4 ). Undernutrition during pregnancy may lead to increased risks of obstetric and neonatal complications including preterm birth, intra-uterine growth retardation and low birth weight, which further increase the risk of adverse health consequences among women and children alike( Reference Black, Victora and Walker 5 ). In lactating women, a poor diet may affect the nutrient quality of breast milk( Reference Innis 6 – Reference Lonnerdal 8 ).
UNICEF’s conceptual framework of malnutrition describes that household food insecurity leads to inadequate dietary intake( 9 ). A few studies have reported associations between poor dietary diversity and food insecurity among reproductive-aged women in resource-poor settings( Reference Campbell, Talegawkar and Christian 10 – Reference Nguyen, Strizich and Lowe 13 ). However, the association has not been studied extensively among pregnant and lactating women (PLW), except for Bangladeshi pregnant mothers( Reference Na, Mehra and Christian 14 – Reference Stevens, Watt and Brimbecombe 16 ).
According to the Malawi Demographic and Health Survey 2015–16, 45·1 % of pregnant women and 29·3 % of lactating mothers are anaemic( 17 ). This condition may be partially due to insufficient dietary intake of Fe( Reference Huddle, Gibson and Cullinan 18 , Reference Munasinghe and van den Broek 19 ). Since the 1990s, the Malawian government has provided free or subsidized input programmes, focusing primarily on maize production among smallholder farmers, prioritizing the poor( Reference Harrigan 20 ). According to a study using data from the Malawi Integrated Household Survey (IHS) 2004/2005 and IHS 2010/2011, per capita food and energy consumption and dietary diversity increased nationwide( Reference Verduzco-Gallo, Olivier Ecker and Pauw 21 ). However, consumption of some key micronutrients declined in rural areas, including vitamin A, folate, Fe and Zn( Reference Verduzco-Gallo, Olivier Ecker and Pauw 21 ).
In the present study we sought to understand how household food insecurity is associated with dietary diversity among pregnant women and lactating women in rural Malawi. Specifically, we examined the association between severity of household food insecurity and PLW’s dietary diversity score (DDS), achievement of minimum dietary diversity (MDD, defined as consumption of three or more food groups in the 24h preceding the survey) and consumption of nine food groups.
Methods
Study setting and population
The present study was conducted in two rural, neighbouring districts in Central Malawi. The main ethnic group in this area of Malawi is Chewa. Most households practise subsistence farming and are dependent on rain-fed agricultural practices. The main staple crops are maize, sweet potatoes and pulses. Predominant cash crops grown are cotton, tobacco and groundnuts( 22 ). Almost 40 % of households experience some type of food insecurity( 23 ).
The present study used the baseline survey data of a large-scale impact evaluation of a stunting prevention programme, based on a cross-sectional pre–post comparison design with one programme and one comparison district. The stunting prevention programme, led by the Government of Malawi and implemented with technical support from the World Food Programme and World Vision Malawi, targeted children 6 to 23 months of age. The programme district was selected based on a high burden of stunting, government policy and partnership for programme scaling up in future( 24 ). In the comparison district, routine government health service was available. PLW with children less than 6 months of age, and children aged 6 to 23 months in the districts, were to be assessed under the programme impact evaluation.
Data collection
The baseline survey was conducted from January to March 2014. A total of 1200 children aged 6 to 23 months and 600 PLW was planned per district for the baseline survey. Assuming 6 % of the total population is children 6 to 23 months of age, the required number of villages to be sampled to reach the sample size was estimated at 108 per district (150–600 households per village). In the selected 216 villages, a total of 12 529 households were screened, out of which 2307 households with pregnant women and lactating mothers were identified. Using a 2/3 sampling ratio, 1296 households with PLW were visited. Pregnancy was assessed verbally during household screening procedures and at the time of the survey. Lactating women with a child less than 6 months of age were identified via maternal report and verified by the child’s date of birth recorded on the health passport/card.
The collected sample size (~600 PLW per district) enabled an 8 % detectable difference in the proportion of women who achieved MDD (defined as consuming at least three food groups out of nine) with 80 % power and α=0·05 from baseline of the intervention to endline.
Interviewers were trained to administer structured household questionnaires using standardized field procedures. Pilot testing was conducted prior to the baseline survey. All questionnaires were translated in the local language, Chichewa. Four teams conducted data collection; each comprised one supervisor, three enumerators, one nurse and one vehicle driver. At the completion of data collection, all forms were transported to a data management centre in Zomba, Malawi. Data were double entered into the Census and Survey Processing System (CSPro) version 3.2 (US Census Bureau, 2007). Data entry errors were identified, verified and corrected against corresponding paper forms.
Measures
Independent variables
Household food insecurity status was measured using a nine-item Household Food Insecurity Access Scale (HFIAS) module that assesses the frequency with which certain aspects of food insecurity are experienced by the household( 25 ). Response options for each item include rarely, sometimes and often (see online supplementary material, Supplemental Table 1). The validity of the HFIAS has been demonstrated in several countries including Tanzania( Reference Knueppel, Demment and Kaiser 26 ), Ethiopia( Reference Gebreyesus, Lunde and Mariam 27 ) and Burundi( Reference Desiere, D’Haese and Niragira 28 ). This scale reflects three universal domains of household-level food insecurity: (i) anxiety about food supply; (ii) inadequate food quality, meaning the lack of food diversity and decreased availability and access to preferred foods; and (iii) insufficient quantity of food, reflected in lower consumption, and the physical consequences of insufficiency( 25 ). Following the standard categorization of HFIAS, the responses were categorized as four levels of household food insecurity: food secure and mildly, moderately and severely food insecure.
Dependent variables
Our dietary assessment questionnaire was based on the FAO guidelines for measuring household and individual dietary diversity( 29 ). The questionnaire included thirteen food items that were surveyed and aggregated into the nine following food groups: (i) grains/roots; (ii) dark green leafy vegetables (DGLV); (iii) vitamin A-rich vegetables or fruits (VAFV) and red palm oil; (iv) other fruits and vegetables (OFV); (v) organ meat; (vi) meat/poultry/fish/insects, (vii) eggs; (viii) beans/lentils/nuts/seeds; and (ix) animal milk and dairy foods. Using this questionnaire, a DDS was computed based on the consumption of foods from nine food groups in the previous 24h. In addition, MDD, which was defined as consumption of three or more food groups out of nine, and the likelihood of consuming foods from nine individual food groups were computed. The threshold of three food groups used for the calculation of MDD was based on the categorization of DDS into 50th upper or lower percentiles. The 50th upper percentile consisted of 3 or more points out of 9, which was used to set the minimum level of dietary diversity of this study group.
Potential confounders
Household- and individual-level characteristics were assessed and explored as potential confounding variables. A priori variable selection was based on theory and previous literature and included demographic and socio-economic variables such as household water sources and sanitation facilities, pregnancy history, education level, occupational status, and health and nutrition service utilization. Household wealth quintiles were created using principal component analysis( Reference Vyas and Kumaranayake 30 ), using twenty-three variables related to ownership of: (i) household assets (number of rooms, electricity, radio, television, bicycle, mobile phones, tape and/or CD player, beds, sofas, tables and chairs, pounding mortar/pestle); (ii) land availability (land ownership, land for rent, land for producing foods, land for producing foods for sale); (iii) any livestock ownership; (iv) years of maternal education; and (v) use of improved toilet facilities. Assets that were surveyed but too infrequent (<1 %) were excluded from the wealth index construction.
Statistical analysis
Exploratory data analyses were conducted. Each variable was presented as mean and sd for continuous variables or n and % for binary/categorical variables. To examine the association between food security and dietary diversity, univariate and multivariable linear or logistic regression models for continuous and binary outcomes, respectively, were conducted. Analyses were conducted separately for pregnant women and lactating women. Potential confounding variables such as district location, household wealth quintiles, drinking-water sources, household size, maternal age, maternal literacy and years of education, maternal occupation, marital status and mid-upper arm circumference were included in multivariable regression models, accounting for village-level clustering. P<0·05 was considered statistically significant. All data were analysed using the statistical software package Stata version 14.
Results
Selection of study participants
Out of 2307 households with PLW identified in 216 villages, 1296 households were visited and 1238 mothers in the two districts completed the survey; eight mothers were excluded, as they were not pregnant or lactating within 6 months after delivery. As a result, 1230 mothers, 589 pregnant women and 641 lactating women, were included in the analysis.
General characteristics of study population
The mean household size was 4·9 (sd 2·1) and 78 % of all households had access to protected drinking-water sources (Table 1). The mean age of PLW was 25·9 (sd 6·5) years; 67 % of them could read and write. A majority (93 %) of mothers were currently married. The primary occupation and main source of income was agricultural farming (83 %). The mothers’ mean mid-upper arm circumference was 26·2 (sd 2·6) cm.
Table 1 Household- and individual-level characteristics of study pregnant and lactating women (n 1230), rural Malawi, January–March 2014

MUAC, mid-upper arm circumference.
Description of household food insecurity and dietary diversity practices
Among pregnant women (n 589), 14·3 % were food secure; 19·0 % experienced mild food insecurity, 23·9 % reported moderate food insecurity and 42·8 % were categorized as severely food insecure. The distribution of household food insecurity among lactating women (n 641) was comparable to that of pregnant women (P=0·74; Table 2).
Table 2 Household food insecurity and dietary diversity of study pregnant and lactating women (n 1230), rural Malawi, January–March 2014

DDS, dietary diversity score; MDD, minimum dietary diversity.
* P values were calculated by χ 2 test.
† DDS measures food consumption in the 24h preceding the survey and is calculated using the nine following food groups: (i) starchy staples (cereals and white roots and tubers); (ii) dark green leafy vegetables; (iii) vitamin A-rich fruits and vegetables; (iv) organ meat; (v) flesh meat, fish and insects; (vi) eggs; (vii) legumes, nuts and seeds; and (ix) milk and dairy products.
Among pregnant women, the mean DDS was low at 3·1 (sd 1·1) and 32·4 % of them met the MDD classification (consumption of three or more food groups in the past 24h; Table 2). Among lactating women, the DDS was 3·0 (sd 1·0) and 28·1 % consumed three or more foods groups in the last 24h.
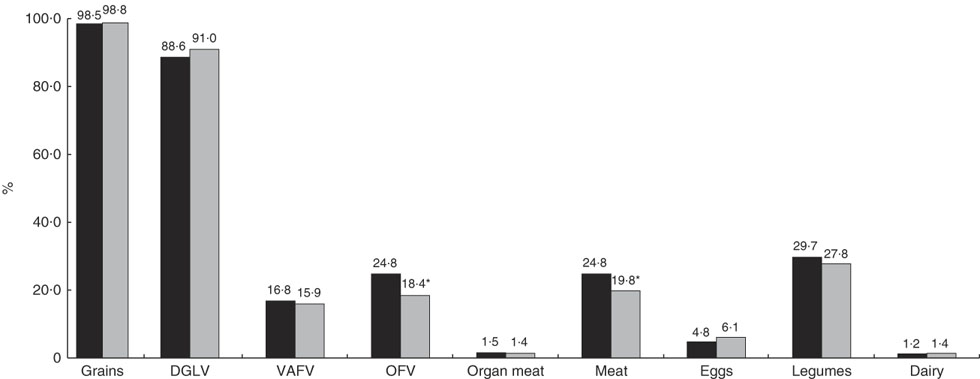
Almost all women consumed grains (98·5 %), most of them had DGLV (88·6 %) and some of them had legumes (28·7 %) in the 24h preceding the survey (Fig. 1). Few of them consumed nutrient-rich food groups such as meat/fish and eggs. Consumption of other types of fruits and vegetables (OFV) than vitamin A-rich ones (24·8 v. 18·4 %; P<0·01) and meat/poultry/fish (24·8 v. 19·8 %; P=0·04) was low but higher among pregnant women than lactating women.

Fig. 1 Food group consumption in the past 24h among study pregnant women (n 589; ![]() ) and lactating women (n 641;
) and lactating women (n 641; ![]() ), rural Malawi, January–March 2014. *P<0·05 (DGLV, dark green leafy vegetables; VAFV, vitamin A-rich fruits and vegetables; OFV, other fruits and vegetables)
), rural Malawi, January–March 2014. *P<0·05 (DGLV, dark green leafy vegetables; VAFV, vitamin A-rich fruits and vegetables; OFV, other fruits and vegetables)
Association between household food insecurity and dietary diversity
Dietary diversity
Compared with food-secure pregnant women, those reporting severe food insecurity had a decrease of 0·36 in DDS (P=0·02). However, those reporting mild or moderate food insecurity did not show a significant difference in DDS, compared with the food-secure pregnant group (Table 3). Compared with food-secure pregnant women, those reporting severe food insecurity reported 2·96 times higher risk of not achieving MDD in the unadjusted model (P<0·01), but the OR was attenuated to 1·83 (P=0·07) when fully adjusted.
Table 3 Association between household food insecurity and dietary diversity among study pregnant and lactating women (n 1230), rural Malawi, January–March 2014

DDS, dietary diversity score; MDD, minimum dietary diversity; Ref., reference category.
* DDS measures food consumption in the 24h preceding the survey and is calculated using the nine following food groups: (i) starchy staples (cereals and white roots and tubers); (ii) dark green leafy vegetables; (iii) vitamin A-rich fruits and vegetables; (iv) organ meat; (v) flesh meat, fish and insects; (vi) eggs; (vii) legumes, nuts and seeds; and (ix) milk and dairy products.
† All linear or logistic regressions were adjusted for district location, household wealth status, drinking-water source, household size, age, literacy, years of education, occupation, marital status and mid-upper arm circumference. Study design effect was accounted for in all models.
Among lactating women, mild, moderate and severe food insecurity was associated with DDS (0·36, 0·44 and 0·62 lower scores, respectively), compared with the food-secure group (Table 3; all P<0·05). Lactating women who reported moderate or severe food insecurity were at 1·95 and 2·82 higher risk of not achieving MDD, respectively, compared with the food-secure group (all P<0·05). However, mild food insecurity was not significantly related to DDS or MDD.
Food group consumption
Compared with food-secure pregnant women, severe food insecurity was associated with threefold (OR=3·19; P<0·01) greater risk of not having meat/fish in the past 24h (Table 4). Also, the risk of not consuming eggs increased by almost four times (OR=3·77; P=0·04) among pregnant women reporting moderate food insecurity. However, the risk of not having other food groups did not differ with severity of food security among pregnant women (see online supplementary material, Supplemental Table 2).
Table 4 Association between household food insecurity and food group consumption among study pregnant and lactating women (n 1230), rural Malawi, January–March 2014

Ref., reference category.
* All logistic regressions were adjusted for district location, household wealth status, drinking-water source, household size, age, literacy, years of education, occupation, marital status and mid-upper arm circumference. Study design effect was accounted for in all models.
The risk of not consuming meat increased with strength of household food insecurity among lactating mothers (Table 4). The risk increased by 1·75, 2·66 and 5·33 times among the mildly, moderately and severely food-insecure group, respectively, compared with the food-secure group (all P<0·05). The risk of not consuming eggs increased by 2·81, 3·75 and 3·47 times among mildly, moderately and severely food-insecure women, respectively, compared with the food-secure group (all P<0·05). The risk of not having legumes increased among only the severely food-insecure group (OR=1·94; P=0·04) but not in the mildly or moderately food-insecure group. The likelihood of not consuming other food groups did not differ with varying household food insecurity among lactating women (see online supplementary material, Supplemental Table 3).
Discussion
Sustainable Development Goal 2 aims to eradicate hunger and all types of malnutrition by 2030( 31 ). To succeed in this goal, ensuring proper nutrition by securing food access and availability to all vulnerable populations, especially PLW, is essential. Our study showed that household food insecurity was associated with low dietary diversity among both pregnant women and lactating women in rural Malawi. While pregnant women’s diet was associated with only severe food insecurity, lactating women were likely to be affected even by mild household food insecurity. The risk of not consuming animal-source foods such as meat/poultry/fish or eggs was high among food-insecure pregnant women and even higher among food-insecure lactating women.
The traditional rural Malawian diet consists of nsima (a maize-based dish), boiled vegetables and relish. Grain-based diets, with low consumption of animal protein and poor intakes of Zn and Fe, were reported among PLW( Reference Gibson and Huddle 32 – Reference Ramlal, Tembo and Soko 34 ). Our study also highlighted the monotonous diet of women in this setting, which is primarily based on grains and DGLV, and is lacking in micronutrients and animal-source protein from animal fleshed foods, dairy products and eggs.
Household food insecurity experience typically begins with worry about food shortages. As this situation worsens, compromises to dietary quality and variety increase. Limiting the quantity of food consumed occurs only under severe circumstances( Reference Radimer, Olson and Campbell 35 , Reference Radimer, Olson and Greene 36 ). In such situations, intra-household food allocation is affected by severe or unexpected food insecurity and women’s diet is often the first to be compromised. Women use their decision-making power or control over resources to maximize family members’, rather than their own, nutritional outcomes( Reference Harris-Fry, Shrestha and Costello 37 ).
In Malawi, food security varies by season. In the dry season (March to August), food availability is usually high, while food shortages are more common in the wet, lean season (September to February)( Reference Chikhungu and Madise 38 ). Given the timing of data collection in the present study (January to March), the percentage of women experiencing moderate or severe food insecurity (68·0 %) would likely be worse than during other times of the year.
The increased risk of not having meat/fish/poultry or eggs implies that the nature of food insecurity in this setting compromises the quality of some diets, given that they are relatively more expensive and nutrient-rich than other foods. Even severe food insecurity among PLW was not associated with reduced consumption of staple foods such as grains and some vegetables (see online supplementary material, Supplemental Tables 2 and 3), which tells us that perhaps staple foods are available to households regardless of whether the household has a sufficient food supply or not. This pattern is consistent with the recent increased availability of grains resulting from strengthened government subsidy programmes that have focused on maize production( Reference Verduzco-Gallo, Olivier Ecker and Pauw 21 ).
The food groups that were associated with severe food insecurity were not always associated with less severe food-insecure conditions (i.e. mildly or moderately). For example, consumption of legumes was associated with moderate food insecurity, but the association was attenuated by more severe conditions among lactating women.
Our study emphasized that household food insecurity and low dietary diversity are problems not only among pregnant but also lactating women in rural Malawi. In the study area, lactating women might be at a higher risk for poor dietary diversity than pregnant women if food insecurity deteriorates. While pregnant women, unless they were in a severely constrained condition, were able to meet a minimum level of dietary diversity, lactating women were at risk of not meeting the MDD even in less severe conditions. Given that the condition of HFIAS was not different between the pregnant women and lactating women (P=0·74, tested by χ 2 test; Table 3), the reasons for varying ability to achieve the lowest dietary diversity between these groups should be examined further. However, we hypothesize that pregnant women may be protected from worsened food insecurity given their upcoming birth, while lactating mothers may be treated as equal to non-lactating women despite their increased nutritional needs to support lactation. If so, continued nutrition education at antenatal and postnatal health visits should not underestimate the importance of a promoting a high-quality diet during lactation.
A handful of studies have examined the association between dietary diversity and household food security or socio-economic status among women, as a proxy indicator for food insecurity in resource-poor settings. In a cross-sectional study of 15 899 women of reproductive age in Nepal, higher socio-economic status was associated with higher consumption of most food groups except for cereal-based foods, fats and edible oils, and flesh food groups( Reference Campbell, Talegawkar and Christian 10 ). Another cross-sectional study of 508 pregnant women in south-western Bangladesh revealed that an economically poorer condition was associated with lower consumption of dairy and eggs( Reference Shamim, Mashreky and Ferdous 15 ). In a study of 161 HIV-negative and 514 HIV-infected Rwandan women, low income was related to food insufficiency and consuming three or fewer food groups, but low BMI (≤18·5 kg/m2) was not related to food insufficiency and dietary diversity( Reference Sirotin, Hoover and Segal-Isaacson 3 ). Only one study examined the association between household food security, as a perception-based quantitative measure, and dietary diversity in a longitudinal cohort following women from pregnancy through lactation in rural Bangladesh( Reference Na, Mehra and Christian 14 ). In that study, higher food insecurity in households was associated with decreased intakes of animal flesh foods, dairy products, eggs and legumes among women. Our study findings are consistent with such findings and contribute to our general understanding of the association between food insecurity and women’s diets in the rural sub-Saharan African setting.
A strength of the present study is the large sample size of PLW. Unlike most of studies that did not distinguish the condition of pregnancy and lactation or targeted only one group, our effort adds detail and richness by comparing the patterns of association between food insecurity and diet profiles of two vulnerable population groups in this setting.
There are a few limitations to our study that deserve mention here. First, our study was not able to adjust for the period of pregnancy and lactation. Last menstruation period was assessed but was not used in our analysis due to limited and unreliable recall by mothers. Instead, we assumed most of the pregnant women interviewed were in their second or third trimester, given the known cultural practice in this context of not disclosing a pregnancy until it is visible( Reference Roberts, Hopp Marshak and Sealy 39 ). Second, given our use of cross-sectional data, we are unable to infer the causal relationship between household food insecurity and dietary diversity. Third, HFIAS is a household-, rather than an individual-level indicator. In addition, some food insecurity domains such as food safety are not assessed. Further, over-reporting for experience of food insecurity may have occurred among some respondents with the expectation of receiving more support in the context of an emergency( 25 ). Fourth, mothers’ diet in the preceding 24h may not be representative of usual consumption patterns. Most mothers were assessed during weekdays, but a few were visited on Saturday. Moreover, the timing during which the study was conducted corresponded with the rainy season, during which dietary patterns differ from those seen in the dry season. Nevertheless, we assumed that the monotonous diet seen in this setting is unlikely to change significantly from one season to another. Lastly, the categorization of foods collected for the study did not allow for the calculation of ten food groups that are required to calculate the Minimum Dietary Diversity for Women (MDD-W) indicator( 40 ). Particularly, out of ten groups that constitute the MDD-W, lentils, nuts/seeds, other fruits and other vegetables were not asked separately in the questionnaire. Also, organ meat was counted as an individual food group in our study, but not an individual food group in MDD-W.
These research findings demonstrate possible evidence of poor dietary diversity attributed to food insecurity, which is experienced by both pregnant women and lactating women in rural Malawi. The findings underline the need to strengthen strategies to promote food security among both pregnant women and lactating women in low-resource settings. For example, food-based activities or conditional cash transfer programmes that were integrated into existing antenatal and postnatal care service improved food security( Reference Nguyen, Kim and Sanghvi 41 , Reference Raghunathan, Chakrabarti and Avula 42 ). Women’s empowerment to increase their control of household financial resources may improve access and utilization of quality foods during pregnancy and lactation( Reference van den Bold, Dillon and Olney 43 ). Also, given the lower consumption of Fe- and protein-rich foods observed among food-insecure PLW in this setting, we could encourage programme practitioners to strengthen protein and micronutrient supplementation efforts or provide food vouchers to these sub-populations.
Conclusion
In conclusion, household food insecurity in rural Malawi was associated with poor dietary practices among PLW. Programme practitioners and policy makers may consider strategies to enhance food security during and after pregnancy when designing nutrition programmes aiming to increase the dietary diversity of this population.
Acknowledgements
Acknowledgements: The authors thank all study participants in the study area, Malawi. They acknowledge the World Food Programme and World Vision Malawi for their guidance and implementation support. The authors appreciate Wadonda Consult Ltd for field data collection and data management. They also thank Audrey Buckland, Johns Hopkins Bloomberg School of Public Health, USA, for the manuscript review. Financial support: This study was funded by Children’s Investment Fund Foundation (CIFF), UK. The funding agency had no role in the design of the study, data collection and analysis, or presentation of the results. Conflict of interest: No authors had conflict of interest related to the study. Authorship: Y.K. and K.M.H. designed the present research; P.C., R.K. and K.P.W. designed a parental trial; A.B.M. and J.P. supervised data collection in Malawi; L.S.F.W. and M.M. managed the data set; Y.K. analysed the data and prepared the manuscript draft; K.M.H., J.R.-B., R.O. and P.C. contributed substantially to interpretation of results; all authors contributed to the writing of the manuscript; Y.K. had primary responsibility for the final content of the manuscript; all authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board at Johns Hopkins School of Public Health, USA and the College of Medicine Research and Ethics Committee (COMREC), University of Malawi, Blantyre, Malawi. The trial is registered to ClinicalTrials.gov (NCT02985359). All subjects were orally consented and informed about the study purpose and scope of assessment.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018002719