Globally, 171 million children (27 %) below 5 years of age were stunted in 2010( Reference de Onis, Blössner and Borghi 1 ). The problem of stunting or chronic undernutrition is deeply rooted in poverty and deprivation, affecting mainly the developing and underdeveloped nations. Over the previous two decades, the prevalence of stunting in Asia has reduced by half from 48·6 % to 27·6 % in the years 1990 to 2010( Reference de Onis, Blössner and Borghi 1 ). In comparison with the global rates, the prevalence of stunting in India is much higher (i.e. 48 %) with the rate in rural areas (50·7 %) exceeding that in urban ones (39·6 %)( 2 ). Stunting in early life is associated with short-term and long-term consequences. Stunting may hamper the structural and functional development of the brain, resulting in delayed cognitive function development or permanent cognitive impairment which may lead to poor academic achievement and low economic productivity( Reference Kar, Rao and Chandramouli 3 – Reference Sokolovic, Selvam and Srinivasan 5 ). Childhood stunting is directly related to short adult stature and may have intergenerational effects. Maternal stunting increases the risk for intra-uterine growth restriction, perinatal mortality, maternal mortality and undernutrition in the offspring( Reference Dewey and Begum 6 ). In addition to this, early stunting is viewed as a risk factor for overweight and adiposity later in life.
Much of the evidence to support the relationship between stunting and obesity emerges from some cross-sectional studies that point towards a possible risk of overweight/obesity among stunted children( Reference Popkin, Richards and Montiero 7 – Reference Pomeroy, Stock and Stanojevic 11 ) and adolescents( Reference Bénéfice, Garnier and Simondon 12 – Reference Santos, Clemente and Martins 14 ). Some studies have also shown that stunted children and adolescents have higher total body fat percentage (BF%) and central adiposity( Reference Santos, Clemente and Martins 14 – Reference Clemante, da Luz Santos and Martins 18 ). Central adiposity has been independently associated with high levels of serum TAG, non-HDL cholesterol, fasting insulin, homeostatic model assessment of insulin resistance and C-reactive protein in young children( Reference Corvalán, Gregory and Ramirez-Zea 19 , Reference Messiah, Arheart and Natale 20 ). Further, Santos et al. ( Reference Santos, Clemente and Martins 14 ) found that adolescents with mild stunting and overweight had significantly higher blood glucose, insulin and homeostatic model assessment of insulin resistance (P<0·05) than normal-weight stunted ones. Also, Kruger et al. ( Reference Kruger, Pretorius and Schette 17 ) showed that stunted girls had higher TNF-α levels compared with non-stunted girls (P=0·03). These findings suggest that stunting at an early age can potentially increase the risk of overweight, glucose intolerance and dyslipidaemia.
To the best of our knowledge, there are no studies that have explored the relationship of stunting and adiposity among Indian children. There is a need to explore this relationship given that India has a high prevalence of stunting in young children( 2 ). Additionally, Indian infants and children possess a ‘thin–fat’ phenotype( Reference Krishnaveni, Hill and Veena 21 , Reference Yajnik, Lubree and Rege 22 ). Adiposity in stunted children depends on the rate of weight gain during the early years. Rapid weight gain during infancy and childhood is a major risk factor for higher BMI, total body fat and central adiposity in later life( Reference Dulloo 23 ). Accelerated weight gain in childhood can be attributed to the rapid nutrition transition and limited facilities for physical activity present in India. Kimani-Murage et al. ( Reference Kimani-Murage, Kahn and Pettifor 9 ) suggested that stunting and obesity may coexist in such transitional societies. All these factors in conjunction with each other predispose stunted children to overweight, thereby increasing the risk for non-communicable diseases.
We therefore conducted a case–control study with the objective to compare the BMI, BF% and waist-to-height ratio (WHtR) of stunted and non-stunted Indian children belonging to low socio-economic background following different growth trajectories.
Materials and methods
The study was approved by the Independent Ethics Committee (IEC no. 09122), Navi Mumbai, Maharashtra, India. Parents and/or guardians of the children received explanation about the study procedure in the local language and written informed consent was obtained from them.
Study design
We conducted a cross-sectional, case–control study in children aged 2–4 years belonging to low socio-economic sections in Mumbai city, India. Initially, we had conducted nutritional assessment of 1200 children aged 2–4 years from July 2013 to July 2014. From this initial group of children, we drew age- and sex-matched stunted (case) and non-stunted (control) children. Stunting was defined as height-for-age Z-score (HAZ) ≤ –2. The non-stunted or control group included children with HAZ ≥ –1·5. After matching for age and sex, the final sample included 330 stunted and 330 non-stunted children.
Participants
Children selected were beneficiaries of anganwadis, which are government-run mother–child care centres in India. Date of birth of the children was obtained from either the anganwadi records or their birth certificates. Age was calculated on the basis of their date of birth to the nearest one month. Birth weight of the participants was obtained from discharge card of the hospital where the child was born or the anganwadi register.
Measurements
All of the following measurements were taken by the researcher in triplicate.
Anthropometry
Weight and height of all children were measured using a standard procedure( Reference Fidanza and Keller 24 ). Children were weighed on a digital weighing scale (model MS8270; Dr Gene Health and Wellness), with an accuracy of 0·1 kg. Height was measured using a non-extensible, flexible measuring tape with an accuracy of 0·1 cm which was calibrated against the standard anthropometric scale. BMI was calculated.
Body fat
Harpenden callipers (Baty International, Burgess Hill, UK) were used to measure the skinfold thickness at triceps (TSF) and subscapular (SSF) sites( Reference Norgan, Fidanza and Sarchielli 25 ). BF% was calculated using the equation given by Slaughter et al. ( Reference Slaughter, Lohman and Boileau 26 ). Slaughter’s equation has been reported to predict BF% with high accuracy (95 %) and minimal bias, and has shown good precision compared with dual-energy X-ray absorptiometry in school-going children in Pakistan( Reference Hussain, Jafar and Zaman 27 ).
Measures of central fat
Waist circumference (WC) and WHtR were used as indicators of central body fat( Reference Van der Kooy and Leenen 28 – Reference Mokha, Srinivasan and Dasmahapatra 30 ). WC has been associated with biomarkers of cardiovascular risk in children( Reference Messiah, Arheart and Natale 20 , Reference Kuriyan, Thomas and Lokesh 31 ). WHtR correlates strongly with BF% and the distribution of body fat( Reference Nambiar, Hughes and Davies 32 ). WC circumference was measured midway between the lowest rib margin (costal margin) and the superior anterior ileac crest with a non-extensible, flexible and accurate tape measure( Reference Seidell 33 ).
Change in weight SD
Change in weight sd was calculated as follows: change in weight sd=present WAZ – WAZ at birth, where WAZ is weight-for-age Z-score. Catch-up growth (CUG), catch-down growth (CDG) and ‘no change in weight sd’ were calculated( Reference Ong, Ahmed and Emmett 34 ). If change in weight sd was >0·67, then it was considered that the child showed CUG. On the other hand, if change in weight sd was <−0·67, then it was considered as CDG. If the change in weight sd was between −0·67 and +0·67, then it was regarded as ‘no change in weight sd’.
Child’s feeding history
Information about breast-feeding – total duration of breast-feeding, whether the child was exclusively breast-fed, duration of exclusive breast-feeding and the age at which complementary feeding was initiated – was obtained from the child’s parent or guardian. In addition, details of income of all the family members were gathered to calculate the total family income.
Statistical analysis
HAZ and WAZ were computed using WHO Anthro software version 3·2·2. The data were analysed using the statistical software package IBM SPSS Statistics Version 20. Mean and sd were calculated for continuous variables and frequency distribution for the categorical variables. One-way ANOVA was performed to see if there were any differences in mean anthropometry, BMI, TSF, SSF, BF%, WC and WHtR between the two groups.
We carried out one-way ANCOVA to study the differences in BMI, BF%, TSF, SSF, WC and WHtR after adjusting the means for certain confounding variables that may potentially influence the growth and nutritional status of the children. The analysis was adjusted for the following covariates: birth weight, change in weight sd, duration of exclusive breast-feeding, total duration of breast-feeding, age at initiation of complementary feeding and total family income. The stunted and non-stunted children were classified into the three categories of change in weight sd: CDG, no change in weight sd and CUG. We performed one-way ANCOVA to study the differences in BMI, BF%, TSF, SSF, WC and WHtR between stunted and non-stunted groups who experienced CDG, no change in weight sd and CUG, independently, after adjusting the means for the studied covariates (birth weight, duration of exclusive breast-feeding, total duration of breast-feeding, age at initiation of complementary feeding and total family income).
Results
Sample profile
The characteristics of the sample are given in Table 1. Fifty-three per cent of the children were girls (n 350) and 47 % were boys (n 310) boys. The stunted group had a greater proportion of low-birth-weight children than the non-stunted group (P=0·001). A higher percentage of stunted children experienced CDG, while more non-stunted children showed CUG and no change in weight sd (P=0·000). In both groups, >90 % of the children received exclusive breast-feeding; however, the total duration of breast-feeding (P=0·008) and that of exclusive breast-feeding (P=0·029) were significantly higher among the stunted than the non-stunted children. The total family income of the stunted children was significantly lower than that of the non-stunted (P=0·008).
Table 1 Characteristics of the sample of children aged 2–4 years from low socio-economic strata in Mumbai, India, July 2013–July 2014
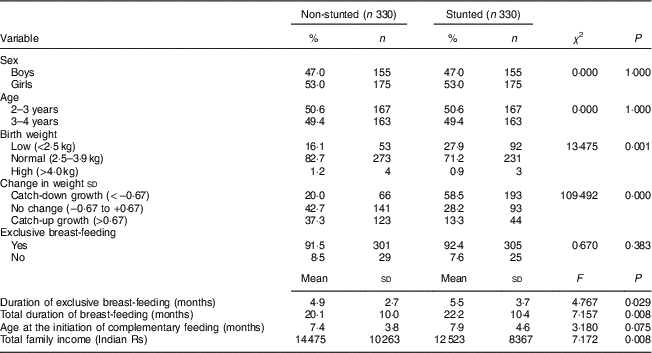
Anthropometry, BMI, body fat percentage, waist circumference and waist-to-height ratio in stunted and non-stunted children
The stunted boys and girls had significantly lower mean birth weight, present weight, height, HAZ and change in weight sd than their non-stunted counterparts (all P<0·05; Table 2). Mean BMI, TSF, SSF, BF% and WHtR were similar between the stunted and non-stunted boys and girls. Only stunted girls had a lower mean WC than the non-stunted girls (P<0·05).
Table 2 Unadjusted mean anthropometric parameters, BMI, skinfold thickness, WC, WHtR, BF% and change in weight sd in stunted and non-stunted boys and girls aged 2–4 years from low socio-economic strata in Mumbai, India, July 2013–July 2014
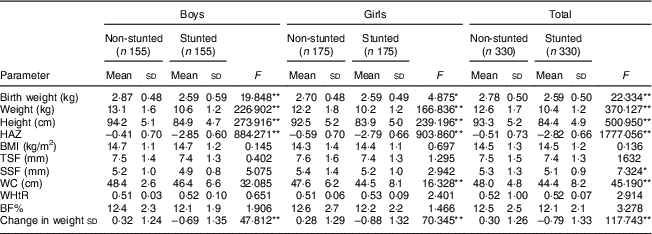
WC, waist circumference; WHtR, waist-to-height ratio; BF%, body fat percentage; HAZ, height-for-age Z-score; TSF, triceps skinfold; SSF, subscapular skinfold.
*P<0·05, **P<0·001.
After adjusting for the covariates (birth weight, change in weight sd, duration of exclusive breast-feeding, total duration of breast-feeding, age at initiation of complementary feeding and total family income), stunted boys and girls were seen to have significantly higher BF%, TSF, SSF, BMI and WHtR than their non-stunted counterparts (all P<0·001; Table 3). Stunted girls had significantly higher WC than the non-stunted girls (P<0·05). However, no such difference was noted in boys for WC.
Table 3 Adjusted mean BMI, BF%, skinfold thickness, WC and WHtR in stunted and non-stunted boys and girls aged 2–4 years from low socio-economic strata in Mumbai, India, July 2013–July 2014
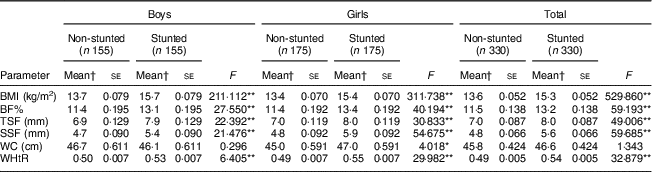
BF%, body fat percentage; WC, waist circumference; WHtR, waist-to-height ratio; TSF, triceps skinfold; SSF, subscapular skinfold.
*P<0·05, **P<0·001.
† Means adjusted for birth weight, change in weight sd, duration of exclusive breast-feeding, total duration of breast-feeding, age at initiation of complementary feeding and total family income.
BMI, body fat percentage, waist circumference and waist-to-height ratio in stunted and non-stunted children who had catch-down growth, no change in weight sd and catch-up growth
After adjusting for the covariates, stunted children who had no change in weight sd had significantly higher mean BF%, BMI (both P<0·001) and WHtR (P<0·05) than their non-stunted counterparts (Table 4). No differences were seen in mean WC measurements among them. In the CDG group, the stunted children had significantly higher BMI (P<0·001) than the non-stunted children. No differences were seen in mean values for BF%, WC and WHtR. On the other hand, in the CUG group, stunted children had significantly higher BMI and WHtR (both P<0·001) than the non-stunted children. Mean BF% was higher in the stunted compared with the non-stunted children but the difference did not reach statistical significance.
Table 4 Adjusted mean BMI, BF%, skinfold thickness, WC and WHtR across the change in weight sd categories in non-stunted and stunted children aged 2–4 years from low socio-economic strata in Mumbai, India, July 2013–July 2014
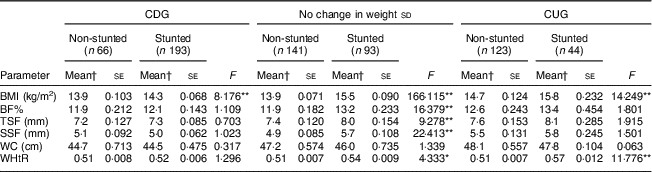
BF%, body fat percentage; WC, waist circumference; WHtR, waist-to-height ratio; CDG, catch-down growth; CUG, catch-up growth; TSF, triceps skinfold; SSF, subscapular skinfold.
*P<0·05, **P<0·001.
† Means adjusted for birth weight, duration of exclusive breast-feeding, total duration of breast-feeding, age at initiation of complementary feeding and total family income.
Discussion
Our study is unique because it represents the first time that the BMI, BF%, WC and WHtR of stunted children who experienced CUG, no change in weight sd and CDG in their early postnatal life have been compared with those of their respective non-stunted counterparts. In our study, almost 28 % of stunted and 16 % of non-stunted children had low birth weight. More than half of the stunted children experienced CDG while one-quarter showed CUG in their postnatal life. After adjusting for the covariates, the stunted children who experienced no change in weight sd had significantly higher BMI, BF% and WHtR than the non-stunted ones. In the CUG group, stunted children had significantly higher BMI and WHtR than the non-stunted ones. Stunted children who had CDG had significantly higher BMI than their non-stunted counterparts.
Our findings of higher BMI, BF% and WHtR in stunted children with no change in weight sd are consistent with previous studies carried out in stunted children( Reference El Taguri, Besmar and Abdel Monem 8 , Reference Bacardi-Gascon, Jimnez-Moran and Santillana-Marin 10 , Reference Pomeroy, Stock and Stanojevic 11 , Reference Hoffman, Martins and Roberts 16 ). Studies have reported that the higher body fat in stunted children could possibly be due to a reduction in the oxidation of fat in these children( Reference Hoffman, Sawaya and Verreschi 35 , Reference Sawaya and Roberts 36 ). The RQ indicates the substrate being used for energy production. In one study, stunted children had a significantly higher RQ (0·92 (sd 0·009)) than control children (0·89 (sd 0·007)). A higher RQ means that the chief substrate for metabolism is carbohydrate (RQ of carbohydrate=1). Stunted children derived 25 % energy from fat oxidation while the controls obtained significantly higher energy (i.e. 34 %) from oxidation of fat( Reference Hoffman, Sawaya and Verreschi 35 ). Evidence also suggests that chronic starvation or undernutrition results in a reduction in total energy expenditure (i.e. RMR, sleeping metabolic rate and physical activity). This is believed to be because the depleted fat stores activate an autoregulatory feedback mechanism that suppresses thermoregulation( Reference Dullo and Jacquet 37 , Reference Dullo, Jacquet and Girardier 38 ). Thus during prolonged starvation or periods of undernutrition the body adapts to lower energy expenditure which improves the metabolic efficiency. Further, Sawaya et al. ( Reference Sawaya, Grillo and Verreschi 39 ) suggested that long-term undernutrition is accompanied by lower circulating levels of insulin-like growth factor-1. Low insulin-like growth factor-1 levels are associated with lower insulin levels and higher ratios of cortisol to insulin. High cortisol levels are associated with central fat deposition. This may explain the higher WHtR found in stunted children than their non-stunted counterparts who experienced no change in weight sd in the present study. Thus, previously undernourished individuals develop a tendency to conserve more body fat particularly, abdominal fat.
We found that stunted children who experienced CUG had higher BMI and WHtR than the non-stunted ones. During recovery from starvation, owing to the improved metabolic efficiency, the body conserves energy and accumulates more body fat. This tendency is referred to as ‘metabolic adaptation’ or ‘poststarvation obesity’( Reference Keys, Brozek and Henschel 40 ). In a prospective study, Grillo et al. ( Reference Grillo, Siqueira and Silva 41 ) found that stunted children gained 6 kg/year while the non-stunted children maintained at 4 kg/year irrespective of both groups consuming similar diets. This was because during the follow-up period there was a lesser increase in RMR but a greater increase in the velocity of weight gain in the stunted group compared with the non-stunted group. The stunted girls had lower body fat at the beginning of the study but gained more fat than the non-stunted ones after 36 months. Thus, infants and children recovering from undernutrition disproportionately accumulate more fat than muscle mass during CUG. In the present study, the stunted children who exhibited CUG had higher mean BF% than the non-stunted group but the difference was not significant.
Stunted children who had CDG had higher BMI than their non-stunted counterparts. However, they did not show much difference in their body fat indices compared with the non-stunted. CDG represents growth faltering or a period of undernutrition. It appears that both stunted and non-stunted children who experienced growth faltering would have equally adapted to lower energy expenditure to improve metabolic efficiency. This may explain the similar BF%, WC and WHtR values in both these groups.
In addition to adaptation, body composition depends on the quality of the diet and the level of physical activity. Although we did not carry out a detailed dietary assessment, surveys carried out in Indian urban low- to middle-income groups have shown an increase in the consumption of energy-dense foods among children( Reference Pingali and Khwaja 42 – Reference Bentley, Das and Alcock 45 ). Bentley et al. ( Reference Bentley, Das and Alcock 45 ) found that in informal settlements of Mumbai, the diets of children under 5 years of age were marked by consumption of unhealthy snacks. This pattern was seen from infancy and increased during childhood years. The low per unit cost of the snacks made it an easier and a more convenient option for parents to include in their children’s daily diet. Researchers have noted a similar unhealthy dietary pattern in overweight/obese stunted children( Reference Popkin, Richards and Montiero 7 , Reference Said-Mohamed, Bernard and Ndzana 46 , Reference Li, Wedick and Lai 47 ). Based on four large nationwide surveys from Russia, China, South Africa and Brazil in children aged 3–6 years and 7–10 years old, Popkin et al. ( Reference Popkin, Richards and Montiero 7 ) noted that stunted children were likely to become overweight or have abdominal obesity when exposed to energy-dense, high-fat foods along with moderate physical activity. Said-Mohammad et al. ( Reference Said-Mohamed, Bernard and Ndzana 46 ) observed that children who were only stunted and those who were stunted plus overweight spent more time in sedentary activities than the only overweight children. Also, the dietary diversity scores of the stunted plus overweight children were significantly lower than those of the only overweight group (P<0·01). Li et al. ( Reference Li, Wedick and Lai 47 ) added that children who were stunted and stunted plus overweight consumed more energy-dense foods and less protein, PUFA, Fe, fruits and vegetables. Sawaya et al. ( Reference Sawaya, Grillo and Verreschi 39 ) observed in mildly stunted girls that baseline fat intake level positively correlated with the rise in weight-for-height after 22 months (P=0·048). This means that stunted girls gained more weight for the corresponding height compared with non-stunted girls.
Contrary to the findings of Sawaya et al. ( Reference Sawaya, Grillo and Verreschi 39 ), Tanner et al. ( Reference Tanner, Leonard and Reyes-Garcia 48 ) reported that adolescents who were stunted at the age of 2 years were less likely to be overweight than those who were never stunted in a longitudinal study in ‘Tsimani’, an indigenous group residing in the Beni Department of Bolivia, South America. These participants belonged to families who earned their living primarily by agriculture and supplemented it with hunting and gathering. They depended wholly on crops grown at household level and domestic animals for food. Also, these children/adolescents actively participated in hunting, farming, fishing and assisting the elders in their work. Thus, diet and physical activity do influence the body composition in stunted children.
India is witnessing a rapid economic, social and nutritional transition. Urban areas are noticing a rise in the population density leading to poor living conditions. At the same time, there has been a shift from traditional foods to the consumption of energy-dense foods rich in fat and carbohydrates. This shift is evident across the socio-economic classes. Indians also possess a thin–fat phenotype from an early age that predisposes them to adverse metabolic outcomes( Reference Krishnaveni, Hill and Veena 21 ). Thus, impoverished living conditions coupled with easy availability of low-cost energy-dense foods and lesser scope for physical activity in areas with high prevalence of stunting elevate the risk of adiposity in Indians during recovery from undernutrition. However, CUG is imperative in stunted children. This CUG should be achieved with a healthy balance between adequate nutrition and physical activity.
Conclusions and recommendations
The present study is one of the first in India to have compared the body fat indices between stunted and non-stunted children belonging to low socio-economic background following different growth trajectories. Our findings suggest that stunted children with no change in weight, CDG and CUG have higher body fat indices than their non-stunted counterparts. Postnatal growth trajectory influences the change in body fat. Urbanization, nutrition transition and poor physical activity can potentially increase the risk of adiposity in stunted children as they grow older. Growth during childhood and adolescence is accompanied by changes in body composition. In view of this, longitudinal studies should be undertaken in India to map the changes in body composition during childhood/adolescence in stunted children across the socio-economic strata. Also, there is a need for preventive measures to reduce the prevalence of stunting. Strategies to increase physical activity and improve dietary quality should be in place to achieve optimal growth without increasing the risk for adiposity in stunted children.
Acknowledgements
Acknowledgements: The authors thank the Independent Ethics Committee, Navi Mumbai, India for granting the ethical clearance to conduct the study. They acknowledge the continuous support and cooperation of the child development project officers, anganwadi workers and helpers. Financial support: This work received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: M.S.S. and P.S.G. conceptualized the study. M.S.S. collected and analysed the data and prepared the manuscript draft. P.S.G. provided critical inputs in the analysis and manuscript preparation. Ethics of human subject participation: The study was approved by Independent Ethics Committee (IEC no. 09122), Navi Mumbai, Maharashtra, India.







