Article contents
XXXVIII.—The Absorption of Light by Inorganic Salts. No. III.: Aqueous Solutions of Nickel Salts in the Visible Spectrum and the Infra-Red
Published online by Cambridge University Press: 15 September 2014
Extract
The methods and the apparatus were the same as in the two previous articles of the series and hence need not be described, the only change being in connection with the galvanometer. Previously it had rested on the table, but when half the results in the infra-red recorded in this paper were obtained, the arrangement was changed; it was suspended by three iron wires each about one and a half metres long from a bracket in the wall and hung with its three levelling screws clearing the table by about one centimetre. Between the table and levelling screws were placed loose wads of cotton wool for the purpose of damping any vibrations that might arise. With this arrangement the zero is very much less sensitive to vibration. With the lamp and scale at one and a half metres—the distance at which they are ordinarily used now–vibrations of the laboratory rarely cause the zero to move 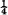 mm. either way.
mm. either way.
- Type
- Research Article
- Information
- Copyright
- Copyright © Royal Society of Edinburgh 1912
References
page 542 note * “Ueber die Lichtabsorption wassriger Lösungen vonKupfer und Nickelsalzen,” Ann. d. Phys. (4), xii. p. 767, 1903.
page 542 note † Nichols, E. L. and Spencer, Mary C., “The Influence of Temperature upon the Transparency of Solutions,” Phys. Rev., ii. p. 344 (1895).Google Scholar
page 542 note ‡ “Untersuchungen über die Absorption des Lichts in Lösungen,” Ann. d. Phys. (4), xxi. p. 575, 1906.
page 542 note § “On a Question in Absorption Spectroscopy,” Proc. Roy. Soc. Edin., xxix. p. 68 (1908).
page 542 note ║
In the former paper the values of c, for which the different values of A are determined) were not given. They are, for copper sulphate ·226, cobalt chloride c=·452, potassium dichromate ·039, and uranyl nitrate ·9906 (last eight points ![]() this strength).
this strength).
page 545 note * Nichols, E. L. and Spencer, Mary C., “The Influence of Temperature upon the Transparency of Solutions,” Phys. Rev., ii. p. 344 (1895).Google Scholar
- 2
- Cited by




