No CrossRef data available.
Article contents
5. The Heats of Combination of the Metals with the Halogens
Published online by Cambridge University Press: 15 September 2014
Extract
Professor Chrystal, in his article on “Electricity” in the Encyclopœdia Britannica, mentions the following formula of Sir William Thomson's, connecting the heats of combination in a voltaic cell with the EMF of the cell.
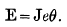
(Where E = EMF of the cell; J = Joule's equivalent; e = amount of zinc, dissolved by unit current in unit time; θ = heat of combination of one grm. of the metal in the cell.)
This formula has been tested for the Daniell and other cells.
It occurred to me that it was peculiarly applicable to the cuprous chloride cell and to the iodine cell recently described to the Society, on account of the simplicity of the reactions in both these cells, and that it could in this way he used as a simple method for the determination of the heats of combination of the metals with the halogens, by determining their electromotive forces, as the active elements in one or other of these cells. Mr. Burton and I accordingly set to work to investigate the matter.
- Type
- Proceedings 1881-82
- Information
- Copyright
- Copyright © Royal Society of Edinburgh 1882




