Article contents
Effect of oxidative stress during imbibition of soybean embryonic axes
Published online by Cambridge University Press: 05 December 2011
Synopsis
Both respiration and 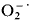 generation by soybean embryonic axes showed a sharp increase upon germination, leading to a significant increase in the steady-state concentration of
generation by soybean embryonic axes showed a sharp increase upon germination, leading to a significant increase in the steady-state concentration of 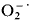 and H2O2 after 6 h of imbibition. An assay was developed to assess in vivo generation of reactive oxygen species, based upon DCFH-DA oxidation. Fluorescence of the external medium was dependent on reaction time and axes number and was inhibited by catalase.
and H2O2 after 6 h of imbibition. An assay was developed to assess in vivo generation of reactive oxygen species, based upon DCFH-DA oxidation. Fluorescence of the external medium was dependent on reaction time and axes number and was inhibited by catalase.
α-Tocopherol content declined significantly after 24 h of incubation, as compared to the content at the onset of germination. Incubation in the presence of redox cycling agent paraquat (4 mM) for 24 h increased α-tocopherol content to 1.9±0.2 nmol per axis from 1.0 ± 0.1 nmol per axis in the absence of paraquat. Supplementation of the incubation medium with 500 μM Fe-EDTA increased α-tocopherol content to 1.8±0.1 nmol/axis and DCFH-DA oxidation by two-fold.
The data presented here showed that active metabolism at the onset of germination increased steady-state concentration of oxygen active species and suggest that cellular content of α-tocopherol is physiologically adjusted as a response to conditions of oxidative stress.
- Type
- Short Communications
- Information
- Copyright
- Copyright © Royal Society of Edinburgh 1994
References
- 1
- Cited by




