This abstract was awarded the Student prize.
Phenolic compounds in plants inhibit α-glucosidase, reducing carbohydrate absorption and contributing to anti-diabetic activity(Reference Lin, Xiao and Zhao1), particularly anthocyanins from coloured fruit and vegetables such as berries(Reference Castro-Acosta, Lenihan-Geels and Corpe2) and black and purple carrots(Reference Esatbeyoglu, Rodríguez-Werner and Schlösser3). The aim of this study was to evaluate inhibition behaviours of an extract from the blue C. ternatea flower against α-glucosidase during in-vitro wheat starch digestion i.e. the potential of the flower extract to modulate starch digestion in a bid to reduce postprandial hyperglycaemic response.
Inhibition of an aqueous extract of dried C. ternatea flowers, at different concentrations, against α-glucosidase was determined according to the method of Sui et al (Reference Sui, Zhang and Zhou4) with minor modifications. In Eppendorf tubes, at each extract concentration, aliquots were mixed with the extract, 0.5 mg/mL α-glucosidase, 40 mg/L calcium chloride, and distilled water to 712 μL, then incubated at 37°C for 15 min for the enzyme to interact with the extract. Aliquots were made up to a 750 μL reaction solution with 1 mg/mL gelatinised starch solution and incubated at 37°C for 5 min to simulate starch digestion. Exactly 100 μL 3,5-dinitrosalicylic acid reagent solution was added to each tube, heated for 10 min in boiling water, then cooled for 10 min in an ice bath; glucose concentration was then measured. Experiments were repeated with C. ternatea extract at 3 mg/mL and differing starch concentrations; 0.5-2 mg/mL. All experiments were repeated in triplicate.
Results showed decreases in glucose liberated and increases in %inhibition, after 5 min digestion, as concentrations of C. ternatea extract increased. Around 80% inhibition was reached at 5 mg/mL C. ternatea extract. Significant statistical differences (p < 0.001) were found between the means of C. ternatea extract groups as determined by one-way ANOVA. Tukey post hoc tests showed that mean glucose liberated between each concentration was statistically significantly lower as concentration increased (p < 0.05), except between concentrations 3 and 4 mg/mL, where the difference found was not statistically significant (p = 0.421). Inhibition activity of C. ternatea extract against α-glucosidase was found to be uncompetitive with KM = 2.309 mg/mL, VMAX = 0.346 mgmin−1 and IC50 = 2.315 mg/mL.

Fig. 1. Effect of C.ternatea extract on % inhibition of α-glucosidase.
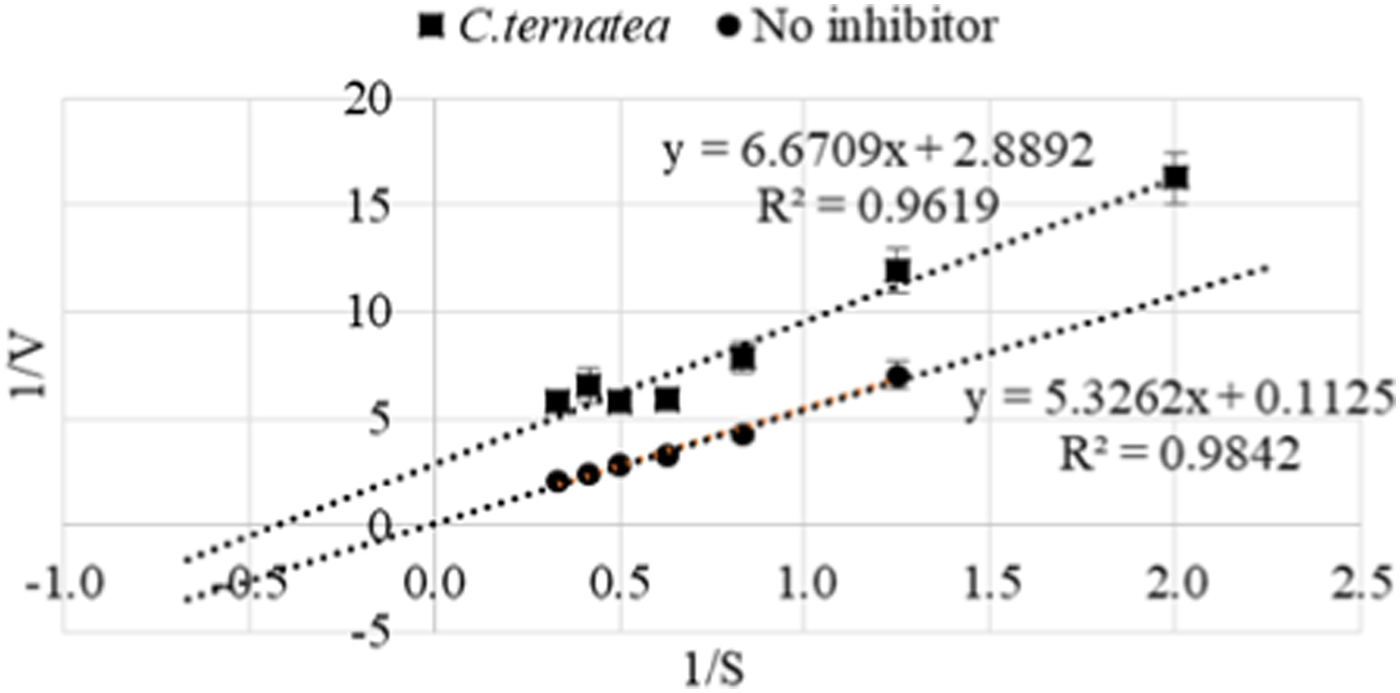
Fig. 2. Lineweaver-Burk plot; with and without C.ternatea extract to assess type of inhibiton against α-glucosidase.
These results suggest that C. ternatea extract has potential to be utilised to prepare functional foods and/or nutraceuticals to help control postprandial hyperglycaemic response therefore, could be used to treat, and reduce the risk of developing, Type 2 diabetes.




