Article contents
Beta diversity, prevalence, and specificity of avian haemosporidian parasites throughout the annual cycle of Chilean Elaenia (Elaenia chilensis), a Neotropical austral migrant
Published online by Cambridge University Press: 27 September 2022
Abstract
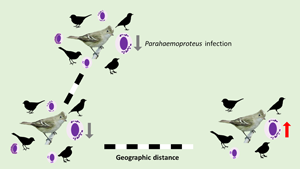
Migratory birds are implicated in dispersing haemosporidian parasites over great geographic distances. However, their role in sharing these vector-transmitted blood parasites with resident avian host species along their migration flyway is not well understood. We studied avian haemosporidian parasites in 10 localities where Chilean Elaenia, a long-distance Neotropical austral migrant species, spends part of its annual cycle to determine local parasite transmission among resident sympatric host species in the elaenia's distributional range across South America. We sampled 371 Chilean Elaenias and 1,818 birds representing 243 additional sympatric species from Brazilian wintering grounds to Argentinian breeding grounds. The 23 haemosporidian lineages found in Chilean Elaenias exhibited considerable variation in distribution, specialization, and turnover across the 10 avian communities in South America. Parasite lineage dissimilarity increased with geographic distance, and infection probability by Parahaemoproteus decreased in localities harbouring a more diverse haemosporidian fauna. Furthermore, blood smears from migrating Chilean Elaenias and local resident avian host species did not contain infective stages of Leucocytozoon, suggesting that transmission did not take place in the Brazilian stopover site. Our analyses confirm that this Neotropical austral migrant connects avian host communities and transports haemosporidian parasites along its distributional range in South America. However, the lack of transmissive stages at stopover site and the infrequent parasite lineage sharing between migratory host populations and residents at breeding and wintering grounds suggest that Chilean Elaenias do not play a significant role in dispersing haemosporidian parasites, nor do they influence local transmission across South America.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
References
- 5
- Cited by



