Introduction
The northern bobwhite quail (Colinus virginianus, hereafter: bobwhite) is a highly popular gamebird in Texas and have a large economic impact on local communities (Johnson et al., Reference Johnson, Rollins and Reyna2012). Although located in various regions throughout the USA (Saunders, Reference Saunders1935; McClure, Reference McClure1949; Pence, Reference Pence1972; Cram, Reference Cram1937), the Rolling Plains ecoregion is one of the last strongholds of bobwhite (Dunham et al., Reference Dunham, Bruno, Almas, Rollins, Fedynich, Presley and Kendall2016a). Bobwhite in this region typically experiences ‘boom and bust’ cycles every 5 years (Hernández and Peterson, Reference Hernández, Peterson and Brennan2007). However, over the past several decades, there has been an average decline of >4% of bobwhite in this area (Sauer et al., Reference Sauer, Link, Fallon, Pardieck and Ziolkowski2013) despite stable habitat conditions (Rollins, Reference Rollins2000, Reference Rollins and Brennan2007). More specifically, in the summer of 2010, the expected ‘boom’ in quail did not occur which ultimately launched Operation Idiopathic Decline (OID) (Dunham et al., Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017a).
OID investigated many different aspects that may have affected bobwhite including parasites, environmental contaminants and habitat conditions. During this collaborative effort in 29 counties of the Rolling Plains, the eyeworm (Oxyspirura petrowi) was identified in 40% of collected bobwhite specimens (Dunham et al., Reference Dunham, Bruno, Almas, Rollins, Fedynich, Presley and Kendall2016a). O. petrowi is a heteroxenous nematode that is usually located under the eyelid and nictitating membrane (Saunders, Reference Saunders1935; Jackson, Reference Jackson1969), inside the lacrimal duct and gland (Robel et al., Reference Robel, Walker, Hagen, Ridley, Kemp and Applegate2003), and in the orbital cavity (Addison and Anderson, Reference Addison and Anderson1969) of their avian hosts (Dunham and Kendall, Reference Dunham and Kendall2017). Further research by Bruno et al. (Reference Bruno, Fedynich, Smith-Herron and Rollins2015) and Dunham et al. (Reference Dunham, Reed, Rollins and Kendall2016b) note pathological findings including inflammation in the lacrimal duct and glands, in addition to lesions on the Harderian gland. These glands are necessary for saturation of the eye (Holly and Lemp, Reference Holly and Lemp1977) and immune defence (Payne, Reference Payne1994), respectively. Additionally, Xiang et al. (Reference Xiang, Guo, Zhang, LaCoste, Rollins, Bruno, Fedynich and Zhu2013) and Kalyanasundaram et al. (Reference Kalyanasundaram, Blanchard, Henry, Brym and Kendall2018a) identified Loa loa as a relative to O. petrowi. This eyeworm is known to infect humans in central Africa and hosts have reported vision impairment and inflammation when infected (Barua et al., Reference Barua, Barua, Hazarika and Das2005; Nayak et al., Reference Nayak, Sinha and Nayak2016). Kalyanasundaram et al. (Reference Kalyanasundaram, Blanchard, Henry, Brym and Kendall2018a) suggest that because of relatives such as L. loa and Thelazia callipaeda, an eyeworm of humans and carnivores, it is not unlikely that O. petrowi could exhibit similar influences on its hosts. This is exemplified in numerous reports of bobwhites being disoriented and colliding with fences, buildings and other stationary objects (Jackson, Reference Jackson1969; Brym et al., Reference Brym, Henry and Kendall2018).
In addition to O. petrowi, the caecal worm (Aulonocephalus pennula), a free-floating nematode of the avian caecum, has been identified in bobwhites of the Rolling Plains with alarming prevalence (Dunham et al., Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017a). Dunham et al. (Reference Dunham, Henry, Brym, Rollins, Helman and Kendall2017b) found evidence of reduced digesta in the caecums of infected bobwhite, suggesting that A. pennula may feed on the digesta of the caecum and thus prevent absorption of necessary nutrients. Additionally, Kalyanasundaram et al. (Reference Kalyanasundaram, Blanchard and Kendall2017) identified Ascardidae, a nematode family containing intestinal nematodes that cause a variety of symptoms in their hosts including weight loss, decreased protein levels, lethargy, and more, as a relative to A. pennula.
Current research suggests these nematode infections may be affected by certain triggers and subsequently influence fluctuations in bobwhite populations in the Rolling Plains. It is understood by Dunham et al. (Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017a) that these parasites can have >90% infection rates depending on environmental conditions. These environmental conditions can include precipitation, temperature, and intermediate host populations. Additionally, nematodes may enter diapause, the temporary halt of growth and development, which can allow them to survive into the next breeding season (Schad, Reference Schad and Esch1977). Nematodes can then exit diapause when environmental conditions permit, thus restarting the life cycle (Sommerville and Davey, Reference Sommerville and Davey2002). The ability to actively test and analyse infection levels in congruence with these various elements would allow a better understanding of what might cause these irruptions in infection rates.
In order to do this, the Wildlife Toxicology Laboratory (WTL) developed the mobile research laboratory as a method for observing these trends in infection. Mobile laboratories have previously been executed for various facets of research including atmospheric pollutant measurements (Bukowiecki et al., Reference Bukowiecki, Dommen, Prevot, Richter, Weingartner and Baltensperger2002), Marburg virus research in Angola (Grolla et al., Reference Grolla, Jones, Fernando, Strong, Stroher, Moller, Paweska, Burt, Palma, Sprecher, Formenty, Roth and Feldmann2011), and human specimen collection and processing for biosafety level two research in Germany (Lermen et al., Reference Lermen, Schmitt, Bartel-Steinbach, Schröter-Kermani, Kolossa-Gehring, von Briesen and Zimmerman2014). Using cloacal swabs in lieu of euthanasia and a highly sensitive multiplex quantitative PCR (qPCR) assay developed by Kalyanasundaram et al. (Reference Kalyanasundaram, Blanchard, Henry, Brym and Kendall2018b) and Kistler et al. (Reference Kistler, Parlos, Peper, Dunham and Kendall2016), the WTL's mobile laboratory can utilize the presence of parasite eggs detected in bobwhite fecal matter to analyse when reproduction is occurring. It is a useful technique that allows large sample sizes to be tested in a time-efficient and cost-effective manner. This could give invaluable insight as to when these parasites may be in diapause and are shedding eggs and ideal treatment times to mitigate them.
A field application was launched in July and August 2017 to test the efficiency of the mobile laboratory in detecting and analysing A. pennula and O. petrowi in bobwhite. The mobile laboratory was deployed in three different regions of the Rolling Plains: Cottle County (upper range), Fisher County (middle range) and Tom Green County (lower range). The objectives of this study are to (1) compare efficiency of collected samples in each of the three areas between a reference laboratory at the WTL and the mobile research laboratory and (2) identify whether mobile laboratory use can identify infection trends among the upper, middle, and lower ranges of the Rolling Plains.
Materials and methods
Ethics statement
This experiment was approved by Texas Tech University Animal Care and Use Committee under protocol 16071-08. All bobwhites were trapped and handled according to Texas Parks and Wildlife permit SRP-0715-095.
Study area and sample collection
In July and August of 2017, bobwhites were collected from three locations throughout the Rolling Plains including Matador Wildlife Management Area (Cottle County), Rolling Plains Quail Research Ranch (Fisher County), and Texas A&M Agrilife Research and Extension Center at San Angelo (Tom Green County) (Fig. 1). The habitat and climate of the overall Rolling Plains study area are as described by Rollins (Reference Rollins and Brennan2007). All bobwhites were collected in the same manner as described in Dunham et al. (Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017a).

Fig. 1. County locations in the Rolling Plains ecoregion of Texas used in field application of mobile laboratory.
Mobile laboratory operation
The mobile laboratory is based out of a 19 × 10 ft2 trailer. One area is dedicated to DNA extractions while the other is strictly dedicated to qPCR so as to avoid contamination. A generator powers the laboratory space to operate machinery at an optimal voltage, with all equipment connected to surge protectors to prevent potential damage.
DNA extraction
Cloacal swab samples were extracted using similar methods described in Kistler et al. (Reference Kistler, Parlos, Peper, Dunham and Kendall2016) using the QIAamp Stool MiniKit (Germany) with a final elution step of 50 µL molecular grade water instead of 200 µL AE Buffer. In total, 75 DNA samples were extracted in the mobile laboratory and 92 DNA samples extracted in the stationary laboratory. Every set of extractions contained 1–2 DNA negatives to account for contamination. All samples were extracted within 1–5 days after collection. When not immediately extracted, swab samples were stored at −20 °C until extraction.
QPCR
The qPCR protocol for this study follows Kalyanasundaram et al. (Reference Kalyanasundaram, Blanchard, Henry, Brym and Kendall2018b). Standards for O. petrowi and A. pennula were used from Kistler et al. (Reference Kistler, Parlos, Peper, Dunham and Kendall2016) and Kalyanasundaram et al. (Reference Kalyanasundaram, Blanchard, Henry, Brym and Kendall2018b), respectively. Standard concentrations used in this study ranged from 105 to 101. All sample DNA and standards were run as duplicates on a StepOnePlus Real-Time PCR system (Thermofisher Scientific) and the results evaluated using StepOnePlus software v2.3 (Thermofisher Scientific).
Statistical analysis
A total of 152 DNA samples were run in the reference laboratory first and divided by extraction location. The same tests were run in the same manner in the mobile laboratory. If duplicates of the sample resulted in one undetermined value and one generated C t value of ≥35, the sample was considered negative. Fifteen samples extracted in the stationary laboratory were removed from the final analysis due to contamination by A. pennula DNA. Additionally, samples from recaptured bobwhite were used in the analysis as well with the assumption of independence based on captures during different trap sessions in addition to varying fecal matter on each sample. Samples were then analysed by per cent similarity between positive and negative samples.
Minitab v7 (USA) was used to generate a P̂ value in addition to 95% confidence intervals for both A. pennula and O. petrowi in the comparison between the mobile and reference laboratories.
Results
Mobile and stationary laboratory comparison
Sample results for A. pennula were similar overall between the reference and mobile laboratories (Table 1). Extractions in the mobile laboratory had the most variation between the reference and mobile laboratories with a three sample difference. A total of 36 samples run in the reference laboratory were considered positive based on returned C t values while a total of 39 samples run in the mobile laboratory were considered positive. Assuming independence for each sample, the comparison between the mobile and reference laboratory efficiency in detecting A. pennula indicated a 97% (P̂ = 0.967) agreement. Ninety-five per cent confidence intervals generated for P̂ were [0.925–0.989].
Table 1. Number and per cent positives for reference and mobile laboratories, separated by extraction location

Similar to A. pennula, sample results for O. petrowi were similar between the reference and mobile laboratories (Table 1). There was little variation between extraction locations when compared with the number of positive samples. Of the samples run in the reference laboratory, nine were considered positive. Of the same samples run in the mobile laboratory, a total of ten samples were considered positive. For O. petrowi, there was a 99% (P̂ = 0.993) alliance between the two laboratories and 95% confidence intervals were [0.964–0.999].
Infection levels
Overall, 25% of individual bobwhites collected in this study were positive for A. pennula by qPCR. About 25% of bobwhites were positive in Cottle County, 26% in Fisher County, and 40% in Tom Green County. Demographics of A. pennula infected bobwhites for each location is visualized in Table 2. Seven per cent (10/143) of individuals were infected with O. petrowi. In Cottle County, 11% were found positive and 5% were found positive in Fisher County. No individuals were found positive for O. petrowi in Tom Green County. Demographic results for O. petrowi are shown in Table 2.
Table 2. Demographic breakdown by location and age class of collected bobwhites
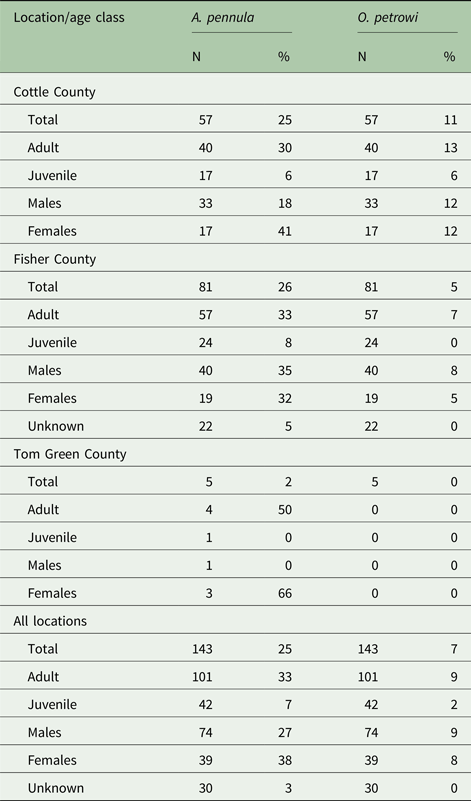
A 95% confidence interval was generated between the per cent positives at each location for A. pennula ([−0.114 to 0.176]) and O. petrowi ([−0.148, 0.037]) to determine any significant difference between locations. Due to Tom Green County's sample size, it was not included in the final analysis. Based on the number of A. pennula (P = 0.694, Fisher's exact test) and O. petrowi (P = 0.318, Fisher's exact test) positives between Cottle and Fisher County, there were no significant differences found in infection levels.
Discussion
Due to its efficiency in detecting the reproductive activity of A. pennula and O. petrowi, the mobile laboratory will provide an accurate and precise method to closely monitor infection of bobwhite throughout the Rolling Plains. With a 97 and 99% efficiency in detecting A. pennula and O. petrowi, respectively, these results suggest that the mobile laboratory has the ability to function similarly to the reference laboratory. Additionally, a large sample size can be assessed for infection in a timely manner with the use of this highly sensitive molecular identification technique.
Of the few variations between laboratories, these samples are suggested to have a low DNA quantity due to their fluctuations of a C t value ≥35 in one duplicate, even after their subsequent reruns. This may be why Kistler et al. (Reference Kistler, Parlos, Peper, Dunham and Kendall2016) and Kalyanasundaram et al. (Reference Kalyanasundaram, Blanchard, Henry, Brym and Kendall2018b) suggest that fecal samples are the preferred method over cloacal swabs in analysing O. petrowi and A. pennula presence. However, it is also suggested by Kistler et al. (Reference Kistler, Parlos, Peper, Dunham and Kendall2016) that swabs can also be used as the preferred method in field applications due to its ease in the collection and reduced stress on the bird. Therefore, the lower C t values observed in this study may correlate to the amount of fecal matter on the swabs. To avoid this, future studies should include a numerical scale given to the amount of feces on the swab to better understand resulting C t values.
The variation in the fecal material on swabs may have contributed to the low infection levels for A. pennula and O. petrowi observed in all three counties, as less than half of each subpopulation collected were infected with these parasites. Low infection levels in Tom Green County, in particular, are likely due to a small sample size. However, it is also important to note that even low amplification of swab DNA could indicate higher infection levels due to the small amount of fecal material present on a cloacal swab. Additionally, the differences between A. pennula and O. petrowi infection can potentially be attributed to the varying numbers of nematodes in an individual. For example, while a bobwhite can have up to 107 O. petrowi adult and juvenile worms (Dunham et al., Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017a), A. pennula numbers can reach a recorded 1162 consisting of adult and juvenile worms (Bruno, Reference Bruno2014). The observed infection levels, while likely influenced by fecal content on the cloacal swab and variable nematode abundance, may also be influenced by temperature, precipitation, diapause and insect intermediate host populations.
Optimal temperature and rainfall have been associated with heightened nematode infections previously (Armour et al., Reference Armour, Jennings and Uruquat1969; Armour and Bruce, Reference Armour and Bruce1974; Lehmann, Reference Lehmann1984; Lima, Reference Lima1998). In order to examine variation in infection levels reported by the mobile laboratory, the climate of these study locations should be monitored in correspondence with the latency periods of O. petrowi and A. pennula. Additionally, it is based on these climatic variations as to when these parasites may enter a stage of diapause (Fernando et al., Reference Fernando, Stockdale and Ashton1971; Michel et al., Reference Michel, Lancaster and Hong1974, Reference Michel, Lancaster and Hong1975, Reference Michel, Lancaster and Hong1976), making it harder to treat these parasites due to reduced energy requirements at this time (Pritchard et al., Reference Pritchard, Donald, Dash and Hennessey1978; Sommerville and Davey, Reference Sommerville and Davey2002). These seasonal factors may also heavily influence the insect intermediate host populations of both parasites. Their population levels can be crucial in understanding subsequent infection levels of their definitive host (Sures and Streit, Reference Sures and Streit2001; Liccioli et al., Reference Liccioli, Kutz, Ruckstuhl and Massolo2014). Consequently, future studies should include observations of the grasshopper Melanoplus sanguinipes, Trimerotropis spp., and species of Gomphocerinae populations as they are identified carriers of A. pennula-infective larvae (Henry et al., Reference Henry, Brym, Kalyanasundaram and Kendall2018).
Of all the infected bobwhites sampled in this field application, most were adults. This is not an unusual occurrence, as Villarreal et al. (Reference Villarreal, Fedynich, Brennan and Rollins2012) and Dunham et al. (Reference Dunham, Soliz, Fedynich, Rollins and Kendall2014) found a higher prevalence of parasites in adult bobwhite when compared with juveniles. However, this could potentially inhibit adult survival in the winter, which according to modelling research is critical to maintaining bobwhite populations (Sandercock et al., Reference Sandercock, Jensen, Williams and Applegate2008). When considering this with the witnessed parasite-induced die-off of bobwhite in the Rolling Plains during spring 2017 that likely resulted from the carry-over infection of winter 2016 (Henry et al., Reference Henry, Brym and Kendall2017), infected adult individuals found in August could impact bobwhite numbers in the following spring given the right environmental conditions. Additionally, while there were no significant trends in infection of males and females in this study, it is possible that the mobile laboratory can identify trends with a larger sample size. This is critical as a previous study of nematodes have reported an effect on fecundity and heightened susceptibility to predators, as exemplified with the extensive research on Trichostrongylus tenuis infection in another galliforme species, the red grouse (Lagopus lagopus scoticus) (Hudson et al., Reference Hudson, Dobson and Newborn1992, Reference Hudson, Dobson and Newborn1998).
In conclusion, the agreement between WTL's mobile and reference laboratories indicates the mobile laboratory's efficiency and accurate detection of O. petrowi and A. pennula. With careful consideration of influencing factors, the mobile laboratory has the ability to determine parasitic activity by reproduction throughout the Rolling Plains. Future applications of the mobile laboratory could also include anthelmintic treatment application based on the timing and intensity of reproductive activity. Most importantly though, the mobile laboratory will allow swift and accurate surveillance of parasitic trends in bobwhite populations across various regions of the Rolling Plains.
Acknowledgements
We thank the employees and volunteers of the Rolling Plains Quail Research Ranch and Matador Wildlife Management Area for allowing access and providing lodging. We also thank Dr James Surles for his help in statistical applications. Lastly, thank you to the Wildlife Toxicology Laboratory personnel for their field and laboratory assistance.
Financial support
We thank Rolling Plains Quail Research Foundation (23A470) and Park Cities Quail (24A125) for their continued financial support of our quail research.
Conflicts of interest
None.
Ethical standards
This experiment was approved by Texas Tech University Animal Care and Use Committee under protocol 16071-08. All bobwhites were trapped and handled according to Texas Parks and Wildlife permit SRP-0715-095.







