Introduction
The Cerrado is the second largest biome in Brazil after the Amazon rainforest and encompasses much environmental heterogeneity at both local and regional scales, characterized by vegetation types dominated by savannah-like ecosystems (Ratter & Dargie, Reference Ratter and Dargie1992; Ratter et al., Reference Ratter, Bridgewater, Atkinson and Ribeiro1996; Klink & Machado, Reference Klink and Machado2005; Silva et al., Reference Silva, Farinas, Felfili and Klink2006). This biome is categorized as a global biodiversity hotspot, with high rates of habitat conversion resulting from recent expansion of soya bean agriculture and cattle ranching threatening many endemic and rare species (Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000; Brooks et al., Reference Brooks, Mittermeier, Mittermeier, Fonseca, Rylands and Konstant2002; Klink & Machado, Reference Klink and Machado2005; Conservation International, 2008).
Attempts to establish conservation priorities for the Cerrado have hitherto been based on subjective criteria (but see Cavalcanti & Joly, Reference Cavalcanti, Joly, Oliveira and Marquis2002; Silva & Bates, Reference Silva and Bates2002; Diniz-Filho et al., Reference Diniz-Filho, Bini, Vieira, Souza, Bastos and Brandão2004, Reference Diniz-Filho, Bini, Pinto, Rangel, Carvalho and Bastos2006, Reference Diniz-Filho, Bini, Rangel, Carvalho, Pinto and Couto2007). In conservation planning, political and economic interests are often given more weight than scientific criteria and thus many protected areas encompass habitat unsuitable for the maintenance of native species. In addition, the lack or misuse of biological information, even when economic aspects are considered, may lead to suboptimal management strategies (Brito, Reference Brito2005). Beyond these common problems, lack of detailed data on species distribution and abundance for most groups of organisms in the Cerrado has discouraged the application of systematic conservation planning (sensu Margules & Pressey, Reference Margules and Pressey2000; but see Diniz-Filho et al., Reference Diniz-Filho, Bini, Vieira, Souza, Bastos and Brandão2004, Reference Diniz-Filho, Bini, Pinto, Rangel, Carvalho and Bastos2006, Reference Diniz-Filho, Bini, Rangel, Carvalho, Pinto and Couto2007; Bini et al., Reference Bini, Diniz-Filho, Rangel, Bastos and Pinto2006).
However, as advocated elsewhere (Diniz-Filho et al., Reference Diniz-Filho, Bini, Vieira, Souza, Bastos and Brandão2004, Reference Diniz-Filho, Bini, Rangel, Carvalho, Pinto and Couto2007), broad scale approaches allow an overview of diversity patterns and thus offer guidelines defined within the framework of conservation biogeography (Whittaker et al., Reference Whittaker, Araújo, Jepson, Ladle, Watson and Willis2005). This hierarchical approach may be particularly useful in poorly known regions that require emergency conservation action because of a combination of high rates of habitat loss and fast human occupation, as is the case with the Brazilian Cerrado.
Patterns of endemism are considered important, explicitly or implicitly, when establishing conservation priorities at multiple spatial scales (Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000), and the Cerrado was categorized as a biodiversity hotspot because of high levels of plant endemism and high threats. These patterns of endemism are related historically to environmental shifts in the region, from savannah-like to dry forests after the Pliocene-Pleistocene transition c. 1.8 million years ago (Silva & Bates, Reference Silva and Bates2002; Werneck & Colli, Reference Werneck and Colli2006). Silva & Bates (Reference Silva and Bates2002) identified three areas of endemism: the Espinhaço Plateau, the Araguaia River valley, and the Paranã River valley, based mainly on overlap of ranges of endemic bird species. Although animal endemism in the Cerrado is relatively low (c. 10% of the terrestrial vertebrates are endemic), there are still > 100 endemic, and mostly rare, species in the region.
In this study we used macroecological data of geographical distributions to evaluate spatial patterns in species richness of endemic terrestrial vertebrates of the Brazilian Cerrado. We evaluate how these patterns can be optimally represented using complementarity-based and irreplaceability procedures, defining which regions of the biome are more important to represent these endemic species. We also find a network that represents all endemics and that, simultaneously, has the minimum amount of human activities within it, based on an evaluation of multiple factors associated with habitat loss. Finally, we evaluate the role of established conservation units in representing these biodiversity patterns.
Methods
Geographical distributions, measured as extents of occurrence (Gaston, Reference Gaston2003), for a total of 1,213 species of terrestrial vertebrates occurring in the core region of the Brazilian Cerrado, were mapped with a spatial resolution of 1o on a grid with 181 cells (Fig. 1; see Diniz-Filho et al., Reference Diniz-Filho, Bini, Vieira, Blamires, Terribile and Bastos2008, for details of sources). We excluded the isolated and peripheral savannah areas in the Amazon region. Although extents of occurrence are an overestimate of species distributions (Araújo, Reference Araújo2004), at broad spatial scales this is the only distributional information that can be used for many regions, and particularly those with high biodiversity and low sampling efforts, such as the Neotropics (Bini et al., Reference Bini, Diniz-Filho, Rangel, Bastos and Pinto2006). Using finer grain sizes may give a false impression of precision and usually increases commission errors (Rondinini et al., Reference Rondinini, Wilson, Boitani, Grantham and Possingham2006). A 1o grid cell is a compromise that recognizes the limitations of the data but may provide guidelines for detailed studies at finer spatial scales (see also Hulbert & Jetz, Reference Hulbert and Jetz2007). As Luck (Reference Luck2007) noted ‘…it is important to use a grain size that has, on average, the potential to incorporate both human settlements and adjacent conservation areas because the impact of humans is not confined within settlement boundaries'.

Fig. 1 Spatial patterns of human occupation in the Brazilian Cerrado, defined by the standardized sum of the first three axes from a factor analyses based on 23 socio-economic variables (Rangel et al., Reference Rangel, Bini, Diniz-Filho, Pinto, Carvalho and Bastos2007; see text for further details). The 181 cells are 1° latitude and longitude.
Species lists from Colli et al. (Reference Colli, Bastos, Araújo, Oliveira and Marquis2002), Macedo (Reference Macedo, Oliveira and Marquis2002) and Marinho-Filho et al. (Reference Marinho-Filho, Rodrigues, Juarez, Oliveira and Marquis2002) were updated, and construction of range maps was based on both primary and secondary literature. Of the 1,213 species mapped, 127 are endemic to the Cerrado (16 species of mammal, 29 birds, 35 reptiles and 47 amphibians; Appendix). A binary matrix was constructed by recording the presence of these species in each cell, and species richness was the sum of the number of species present in each cell.
Based on the occurrence matrix for the 127 endemic species in the 181 cells we used optimization procedures to select the minimum number of cells necessary to represent all species at least once (Church et al., Reference Church, Stoms and Davis1996; Pressey et al., Reference Pressey, Possingham and Day1997; Possingham et al., Reference Possingham, Ball, Andelman, Ferson and Burgman2000; Polasky et al., Reference Polasky, Camm, Solow, Csuti, White and Ding2000, Reference Polasky, Csuti, Vossler and Meyer2001; Cabeza & Moilanen, Reference Cabeza and Moilanen2001), the so-called set-covering problem. A simulated annealing algorithm was used to achieve this. It begins with a random set of cells and, for each iteration, swaps sites in and out of that set, measuring the change in cost according to a cost function. The optimization procedure was repeated 100 times, and final networks were obtained after 1,000,000 iterations, implemented in the Site Selection Mode routine of the software SITES (Possingham et al., Reference Possingham, Ball, Andelman, Ferson and Burgman2000). A relatively high penalty value of losing a species was set so that all solutions tended to represent all species with a minimum number of cells. Given a large number of possible planning units (cells in our case) within the region of interest, SITES selects those units that, simultaneously, meet the conservation goals (i.e. to represent all species at least once) and minimize cost (by selecting a minimum set of planning units).
Multiple solutions satisfying this representation goal can be obtained by Site Selection Mode and, in this case, they were combined to generate a map that gives the relative importance of each cell in achieving minimum networks. We calculated the frequency of cells across the 100 minimum solutions found by Site Selection Mode as an estimate of the irreplaceability of the cell (Ferrier et al., Reference Ferrier, Pressey and Barrett2000; Meir et al., Reference Meir, Andelman and Possingham2004). This irreplaceability ranges from 0 (minimum irreplaceability) to 100% (maximum irreplaceability), measuring the likelihood that a given cell will need to be protected to ensure all species are represented at least once. A cell with a species that occurs only there will have maximum irreplaceability because there is no chance to conserve this species elsewhere.
We also added a cost for each cell based on 23 variables expressing variation in agricultural, demographic and cattle-ranching patterns in the Cerrado (IBGE, 2008; see Table 1 of Rangel et al., Reference Rangel, Bini, Diniz-Filho, Pinto, Carvalho and Bastos2007, for the complete list of variables). Firstly, data entering each cell were calculated by summing or averaging data from 1,054 municipalities whose geopolitical limits lie within the Cerrado's borders. Secondly, we performed a factor analysis (Legendre & Legendre, Reference Legendre and Legendre1998) on the resultant matrix (23 variables × 181 cells) to reduce the dimensionality of the data and to indicate independent patterns of human occupation in the Cerrado. We used a varimax rotation of scores to minimize the number of variables that have high loadings on each factor and, in this way, simplify their interpretation. We also used the Broken-Stick model as a criterion to retain PCA axes for interpretation: a principal component (axis) is retained if its associated eigenvalue is larger than the value given by a broken-stick null distribution of eigenvalues (see Legendre & Legendre, Reference Legendre and Legendre1998, and Peres-Neto et al., Reference Peres-Neto, Jackson and Somers2005, for a detailed explanation). Of three interpretable axes (see Rangel et al., Reference Rangel, Bini, Diniz-Filho, Pinto, Carvalho and Bastos2007), the first factor axis was positively correlated with variables indicating modern agriculture activities (intensive use of measures to control arable soil erosion, high per capita incomes, high levels of investment in pesticides and biological control, fertilization, and farming machinery, associated with relatively low human population fecundity rates). Variables related to cattle ranching were highly correlated with the second axis (pasture land used for cattle ranching, and bovine herd size). The last interpretable axis was positively correlated with total human population, percentage of population that is rural, and high number of farms with areas < 100 ha (a surrogate of agricultural landscape fragmentation; see Rangel et al., Reference Rangel, Bini, Diniz-Filho, Pinto, Carvalho and Bastos2007, for further details).
Thus, Site Selection Mode was run to minimize costs and at the same time force all species to be represented in at least one cell. These costs were expressed both by each factor analysis axis (reflecting different underlying cost assumptions for cattle ranching, modern agriculture or human population density; Rangel et al., Reference Rangel, Bini, Diniz-Filho, Pinto, Carvalho and Bastos2007) and by the overall sum of these scores, expressing simultaneously multiple dimensions of human occupation in the Cerrado (Fig. 1). Thus, high values of this combined cost indicate cells with high incidence of modern agriculture, cattle-ranching activities or human population density. Previous studies have used only total human population in optimization models, finding the network that represents all species but has the minimum human population (Chown et al., Reference Chown, van Rensburg, Gaston, Rodrigues and van Jaarsveld2003). However, because of the mode of recent human occupation in the Cerrado, based on rapid expansion of highly technological agriculture and extensive cattle-ranching practices (Klink & Machado, Reference Klink and Machado2005), more complex socio-economic patterns (e.g. cattle ranching and land conversion for soya bean production are not associated with high human population density) need to be included. This allowed us to choose, amongst many possible network solutions, the one that represents all species and whose regions (i.e. cells) are located where there is the smallest possible total human occupation in respect to all these axes of human occupation (sensu Balmford et al., Reference Balmford, Moore, Brooks, Burgess, Hansen and Williams2001; Araújo, Reference Araújo2003; see also Diniz-Filho et al., Reference Diniz-Filho, Bini, Pinto, Rangel, Carvalho and Bastos2006).
We performed an evaluation of the efficiency of the conservation system already implemented in the Cerrado in terms of its ability to represent the endemic terrestrial vertebrates. Firstly, we included in our analyses only the 33 units of protection > 10,000 hectares, which are in 26 cells. We consider a cell is protected if it includes at least one of these large units, and we counted the proportion of the species that were covered by these units (Rodrigues et al., Reference Rodrigues, Andelman, Bakarr, Boitani, Brooks and Cowling2004). We then used a randomization test to evaluate if the number of species represented in these 26 cells is different from the values expected by chance alone. To do this we counted the proportion of species covered by 10,000 networks with 26 cells randomly allocated (Chown et al., Reference Chown, van Rensburg, Gaston, Rodrigues and van Jaarsveld2003; O'Dea et al., Reference O'Dea, Araújo and Whittaker2006). Secondly, we repeated the Site Selection Mode analysis after fixing the protected cells. Thus, Site Selection Mode was used to add more cells to the initial configuration of 26 cells, where necessary, to represent all species in the final system. For instance, if all endemic species are already represented in these 26 initial protected cells, no further increases would be necessary.
Results
The highest richness of endemic species was found in the central-southern region of the Cerrado, decreasing towards the north-east (Fig. 2). Of the 127 endemic species analysed, 19 occurred in a single cell, and 35 were found in only one or two cells. Thus, the overall range size frequency distribution follows the well-known skewed pattern in which most species are restricted to a few cells (Fig. 3; Gaston, Reference Gaston2003). Because of small geographic ranges, a relatively large number of cells are required to represent all species.
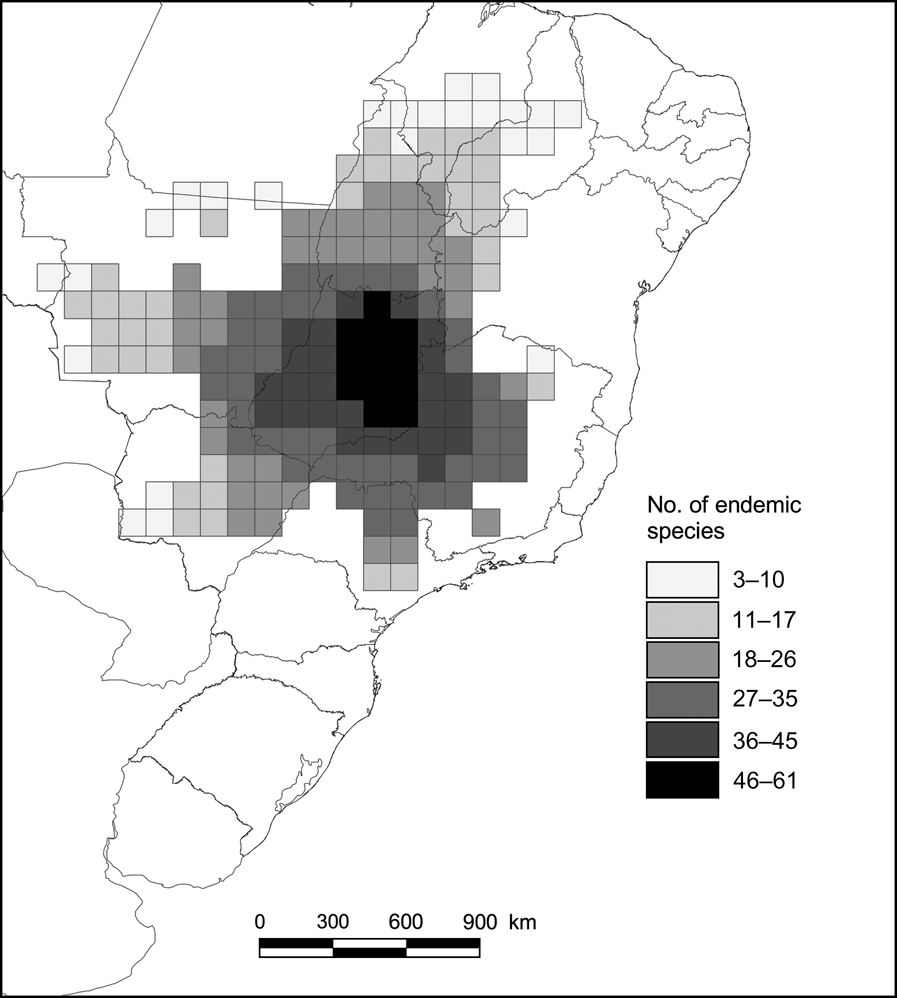
Fig. 2 Number of endemic species of vertebrates (of a total of 127) in each of the 181 1° latitude and longitude cells of the Brazilian Cerrado.

Fig. 3 Frequency distribution of species' range sizes in terms of the 181 1° latitude and longitude cells of the Brazilian Cerrado (Fig. 2).
The Site Selection Mode analysis indicated that 24 cells are required to represent all 127 endemic species at least once. Because of the patterns of extents of occurrence and beta diversity, the levels of representation of most species provided by this network were higher than the minimum target required from the simulated annealing procedure (Fig. 4). For example, 66.9% of the 127 endemic species were represented more than once in the simulated annealing network and, therefore, they were represented in different areas within their ranges; 14.9% were represented only once, mainly because they are already restricted to a single cell in the grid.

Fig. 4 Rank of representation of the endemic terrestrial vertebrate species of each group (mammal, bird, reptile and amphibian) in the network of 24 1o cells that minimizes overall human occupation in the Brazilian Cerrado. Representation is given by the number of cells in the network occupied by the species divided by its total range within the Cerrado.
The frequency of cells across all 100 solutions (i.e. the irreplaceability) was usually low, with only 14 cells being considered highly irreplaceable (Fig. 5). The overall pattern of irreplaceability values reveals that important regions for species representation are widely distributed across the entire Cerrado, although cells with maximum irreplaceability (i.e. those that appear in all 100 solutions) were concentrated in the south-east.

Fig. 5 Spatial patterns of irreplaceability, given by the frequency of 1° cells of the Brazilian Cerrado in the 100 minimum solutions found by Site Selection Mode, which measures the likelihood that a given cell will need to be protected to ensure all species are represented at least once (see text for details).
Despite differences in spatial patterns of human occupation generated by the three factor axes, the site selection solutions found that minimizing each one is similar (Fig. 6). This is expected because most species occur in one or two cells (Fig. 3), restricting the potential representation solutions and decreasing the degree of flexibility of the site selection process. In these solutions, cells are allocated preferentially in the south and south-east, with fewer cells in the north-east, as expected from the irreplaceability patterns.

Fig. 6 Spatial patterns of selected 1° cells of the Brazilian Cerrado in the Site Selection Mode analysis (see text for details) that represent all endemic terrestrial vertebrates and that, at the same time, (a) minimize overall human occupation (i.e. the standardized sum of the three PCA axes), (b) minimize land use by modern agriculture (i.e. the first PCA axis), (c) cattle ranching (i.e. the second PCA axis), and (d) human demography (i.e. the third PCA axis).
Randomly selecting 26 cells across the biome allows representation of 89 ± SE 6 species (Fig. 7), which is significantly less than the observed representation of 101 species. The chance of representing these 101 species (80%) using 26 cells by chance alone is very low (P = 0.018), suggesting that current location of protected cells is not random with respect to endemism. Despite this relatively high efficiency, when we fixed these cells in the Site Selection Mode analysis, the total number of cells needed to represent all species increases to 43 widely distributed in the biome (Fig. 8).

Fig. 7 Frequency distribution of number of represented species in 10,000 randomly generated networks with 24 1° cells, showing the low efficiency of species representation with a randomly defined network.

Fig. 8 Site Selection Mode analysis after fixing the 26 cells of the Brazilian Cerrado that already contain conservation units in the final solution, maximizing species representation and minimizing human occupation. Grey cells were selected by Site Selection Mode, black cells already contain conservation units, and hatched cells indicate overlap between them.
Discussion
Spatial patterns in the richness of endemic birds of the Cerrado were described and analysed by Silva & Bates (Reference Silva and Bates2002) with respect to historical processes of broad scale environmental transitions between savannah-like and forest habitats since the Pliocene-Pleistocene transition (see also Silva, Reference Silva1997). The three areas of endemism they recognized were all inside (or are close to, as with the Araguaia River valley) the area of high richness found in this study for all vertebrates, in the central south-east of the Cerrado. It is not currently possible to differentiate areas within this general area more clearly because the extents of occurrence used in this study tend to overestimate areas of species occurrence and increase commission errors. However, there is a paucity of faunal inventories, especially for reptiles and amphibians, in the northern Cerrado (Bini et al., Reference Bini, Diniz-Filho, Rangel, Bastos and Pinto2006) and thus further inventories would probably expand the pattern of endemic areas northwards (Bini et al., Reference Bini, Diniz-Filho, Rangel, Bastos and Pinto2006).
The patterns of endemic vertebrate species richness of the Cerrado are similar to expectations of the so-called mid domain effect proposed by Colwell & Lees (Reference Cowell and Lees2000; see Hawkins & Diniz-Filho, Reference Hawkins and Diniz-Filho2002; Zapata et al., Reference Zapata, Gaston and Chown2003; Colwell et al., Reference Colwell, Rahbek and Gotelli2004; Hawkins et al., Reference Hawkins, Diniz-Filho and Weis2005, for discussions), in which random overlap of ranges creates high richness in the middle of a study area. However, under certain conditions, the patterns resulting from a mid domain effect could be a result of stochastic dispersion and local extinction, as described by Hubbell's (Reference Hubbell2001) neutral dynamics (Rangel & Diniz-Filho, Reference Rangel and Diniz-Filho2005). In Hubbell's model richness patterns are generated by a random balance between aggregation of species due to distance-mediated dispersal and random extinction in local sampling units. Further investigations of this issue are necessary, especially linking potential mid domain effect patterns and processes of habitat shifts in a historical context (Silva & Bates, Reference Silva and Bates2002). However, there is strong sampling bias in the Cerrado, with a concentration of researchers in the centre of the biome (in the urban centres of Brasilia and Goiânia), which could artificially generate a mid domain effect.
Despite the difficulties of inferring evolutionary (i.e. processes that occurred during the Pliocene-Pleistocene transition) and ecological (i.e. current stochastic processes of dispersion and local extinction) mechanisms to explain richness patterns in the Brazilian Cerrado, the urgency of conservation issues in the biome dictates that conservation planning must be performed using currently available knowledge. Here we defined reserve networks by optimal solutions based on extents of occurrence, which provide a general description of currently known distributional patterns. Although this approach is likely to generate high levels of commission errors (Gaston & Rodrigues, 2002) there are advantages to using it because of the relatively low level of available knowledge of the distribution of the vertebrates of the Cerrado. Also, under a conservation biogeography framework (Whittaker et al., Reference Whittaker, Araújo, Jepson, Ladle, Watson and Willis2005), and using relatively large grain sizes (i.e. 1o of latitude and longitude), commission errors tend to be at an acceptable level (Rondinini et al., Reference Rondinini, Wilson, Boitani, Grantham and Possingham2006). This is especially true if one considers that our main purpose is to define a hierarchical approach for conservation planning that starts at a broad, regional scale and allows further, more detailed investigations and development of planning strategies at a finer spatial scale (Diniz-Filho et al., Reference Diniz-Filho, Bini, Vieira, Souza, Bastos and Brandão2004, 2007). An alternative approach to deal with lack of detailed inventories is to use niche-based modelling strategies to define distribution patterns better (Araújo & Guisan, Reference Araújo and Guisan2006; Araújo & New, Reference Araújo and New2007), which in turn can be used for conservation planning. However, most of the endemic species of the Cerrado are recorded in only a few places, making it difficult to apply these techniques in a systematic way.
Our analyses showed that conservation efforts for representing endemic terrestrial vertebrates of the Brazilian Cerrado should be concentrated in at least 24 cells of 1o latitude and longitude, covering seven states (Goiás, Minas Gerais, Tocantins, Bahia, Maranhão, Mato Grosso, and Mato Grosso do Sul). Because we used extents of occurrence and expected patterns of beta diversity, multiple solutions to represent all species are always available, giving a certain level of flexibility in the system, although many areas with maximum irreplaceability are concentrated in the central and south-east. This is expected because solutions are constrained, in geographical terms, by a number of small-range endemic species (mainly anurans) whose distributions tend to be concentrated in this area.
In Brazil large biomes have been considered as broad scale targets for conservation actions, and in the case of the Cerrado this is reinforced by its status as a global biodiversity hotspot (Cavalcanti & Joly, Reference Cavalcanti, Joly, Oliveira and Marquis2002). An important outcome of our analyses is that to consider the Cerrado biome as a unique and coherent unit it will be necessary to establish a national geopolitical coordination for conservation planning to minimize the loss of efficiency that would occur if conservation areas were to be independently established in each state (Rodrigues & Gaston, Reference Rodrigues and Gaston2002). Even more importantly, the geographical coverage of the conservation units already established in the biome overlap c. 80% of the endemic vertebrate ranges. On the one hand, this is a conservative measure because we only considered conservation units > 10,000 ha, and small units can also protect species. On the other hand, our macroecological analysis does not ensure that species are found within conservation units, both because these are usually much smaller than our grain size and because we are not able to analyse if each conservation unit has suitable habitat for each species. Also, our Site Selection Mode analysis shows that, even though a high proportion of endemic species is represented by the current system of protected areas, the remaining species would require a large number of new areas to be protected. Thus, although geographical gaps are relatively small when data is analysed at broad geographical scales, our preliminary conservation biogeography approach supports previous claims that conservation throughout this biome needs to be improved by adding more protected areas (Silva & Bates, Reference Silva and Bates2002).
We included in our optimization models a variable (sum of the scores of the first three factor analysis axes) that comprises different types of human activities but that is statistically independent. Thus, the selected areas (Fig. 6) are those with the lowest human population densities and are also unsuitable for agriculture and cattle ranching, which are, in general, the main factors that threaten biodiversity in the Cerrado (Klink & Machado, Reference Klink and Machado2005; Rangel et al., Reference Rangel, Bini, Diniz-Filho, Pinto, Carvalho and Bastos2007). Nevertheless, the Site Selection Mode solutions that we used are always similar under the alternative scenarios expressing human occupation because of the restricted range of endemic Cerrado species. Conservation efforts should therefore be targeted independently of the type of human activities that are expected to threaten diversity.
Our analysis provides guidelines for future conservation and research programmes for defining priority regions for conservation in the Brazilian Cerrado. The next step will be to generate more detailed species distributions at multiple spatial scales, using niche-based modelling approaches. More intensive and detailed local sampling within each region can then be used to ensure the persistence and long-term maintenance of important areas for conservation and, at the same time, facilitate a better understanding of patterns of richness and endemism. We anticipate that our findings can drive conservation planning by guiding and coordinating sampling efforts at local scales and, after such validation and refinement, by advising government authorities and decision makers where it will be more efficient to purchase land that meets a given conservation goal.
Acknowledgements
Financial support for this study came from a PRONEX programme of the Conselho Nacional de Desenvolvimento Cientifico (CNPq) & Tecnológico and Secretaria de Ciência & Tecnologia (no. 23234156). JAFDF, LMB and RPB were also partially supported by other CNPq projects (nos 300762/94-1, 300367/96-1, 400381-97.4), and NMT, GO, LCT, TFLVBR, MPP and PC were supported by Coordenação para Aperfeiçoamento de Pessoal de Ensino Superior graduate fellowships.
Appendix
The appendix for this article is available online at http://journals.cambridge.org
Biographical sketches
This paper is part of a Conselho Nacional de Desenvolvimento Cientifico & Tecnológico-PRONEX project to develop and apply optimization and spatial methods to find conservation priority areas in the Cerrado region of central Brazil, coordinated by José Alexandre Felizola Diniz-Filho, Luis Mauricio Bini and Rogerio Pereira Bastos of the Universidade Federal de Goiás. The team of authors included PhD and MSc students in ecology, evolution, animal biology and environmental sciences (Míriam Plaza Pinto, Levi Carina Terribile, Guilherme de Oliveira, Bruno de Souza Barreto, Priscilla Carvalho, Thiago Fernando L.V.B. Rangel and Natalia Mundin Tôrres) and Cleiber Marques Vieira and Daniel Blamires, who now work at the Universidade Estadual de Goiás. All members of the team brought their various areas of expertise and taxonomic knowledge to this study.












