Introduction
The increasing global food demand, strictly linked to the growing world population, expected to reach almost 10 billion in 2050, has brought a new global interest on the human consumption of edible insects(1). The consumption of insects by humans is not a novel phenomenon, having been practiced since early in human evolution, and is nowadays part of the usual diet in many countries(Reference de Carvalho, Madureira and Pintado2). Edible insects have positive environmental and economic implications and are characterised by high feed-conversion efficiency and low greenhouse gas (GHG) emissions, land use and environmental contamination(1). Recently, consumption of edible insects has been widely suggested as an environmentally sustainable substitute for meat and animal products to reduce their extremely high global intake, responsible for massive GHG emission(1).
From a nutritional point of view, edible insects are a good source of nutrients characterised by high levels of essential amino acids, fibre, vitamin B12, iron, zinc, omega-3 and omega-6 fatty acids, and polysaccharides and might contribute to a balanced gut microbiota(Reference Ercolini and Fogliano3). However, the innovative aspect of edible insects is related to their content of bioactive ingredients and their role as functional foods, defined as foods providing a health benefit beyond basic nutrition.
Throughout human history, entomotherapy, the medicinal use of edible insects, has been used in traditional medicine in wound healing and as curative therapy for respiratory disorders and stomachache(Reference Costa-Neto4). The available body of evidence on the functional role of edible insects is mainly focused on their role as antioxidant ingredients in vitro or in cellular models, as recently reviewed by D’Antonio et al. (Reference D’Antonio, Serafini and Battista5). Moreover, there is scattered evidence on the role of edible insects in platelet aggregation, as anti-inflammatory agents(Reference Hwang, Chang and Lee6) or as modulators of lipid(Reference Luo, Wang and Lv7) and glucose metabolism(Reference Zhao, Wang and Wei8). Furthermore, the available studies tested a wide range of different insects, extracts or protein fractions, making it difficult to draw clear conclusions about their functional properties without a review of the available evidence. Recently, findings related to the nutritional, functional and health properties as well as consumer acceptance of edible insects have been thoroughly reviewed(Reference Nowakowski, Miller and Miller9–Reference Stull13). However, none of the manuscripts provided a comprehensive and systematic review of the available evidence on the functional roles of edible insects.
The goal of this work is to review the available body of evidence on the properties of edible insects in modulating oxidative and inflammatory stress, platelet aggregation, weight control, and lipid and glucose metabolism.
Material and methods
The search strategy of the study is shown in Figure 1. We first systematically searched PubMed database (National Library of Medicine, Bethesda, MD; https://pubmed.ncbi.nlm.nih.gov) using the following keywords: (edible insect) AND (functional) OR cardiovascular) OR platelet aggregation) OR oxidative) OR antioxidant) OR glycemia) OR diabetes) OR inflammation) OR immune) OR cholesterol) OR hypolipidemic) OR hypocholesterolemic) OR thrombosis) OR hepatoprotective) OR liver) OR adipose tissue) OR body composition) OR hypoglycaemic). The search, carried out in November 2020, with no limit ranges for the year of publication and with the ‘English’ filter activated, yielded 620 results (Figure 1). Reviews, systematic reviews and meta-analyses (n = 78) were excluded. Articles were screened to exclude those not relevant to the topics such as allergenic properties, nutrient composition, technological functionality, safety assessment, rearing, non-edible insects or other functional effects (n = 492). The references of the selected papers were screened, and two additional papers were identified. Furthermore, to include papers without edible insect as key word, we performed a search for the following insect names: Bombyx mori, Tenebrio molitor, cricket and grasshoppers, identifying three more papers. Fifty-five papers, focusing on oxidative and inflammatory stress, platelet aggregation, weight control, lipid and glucose metabolism, were identified.
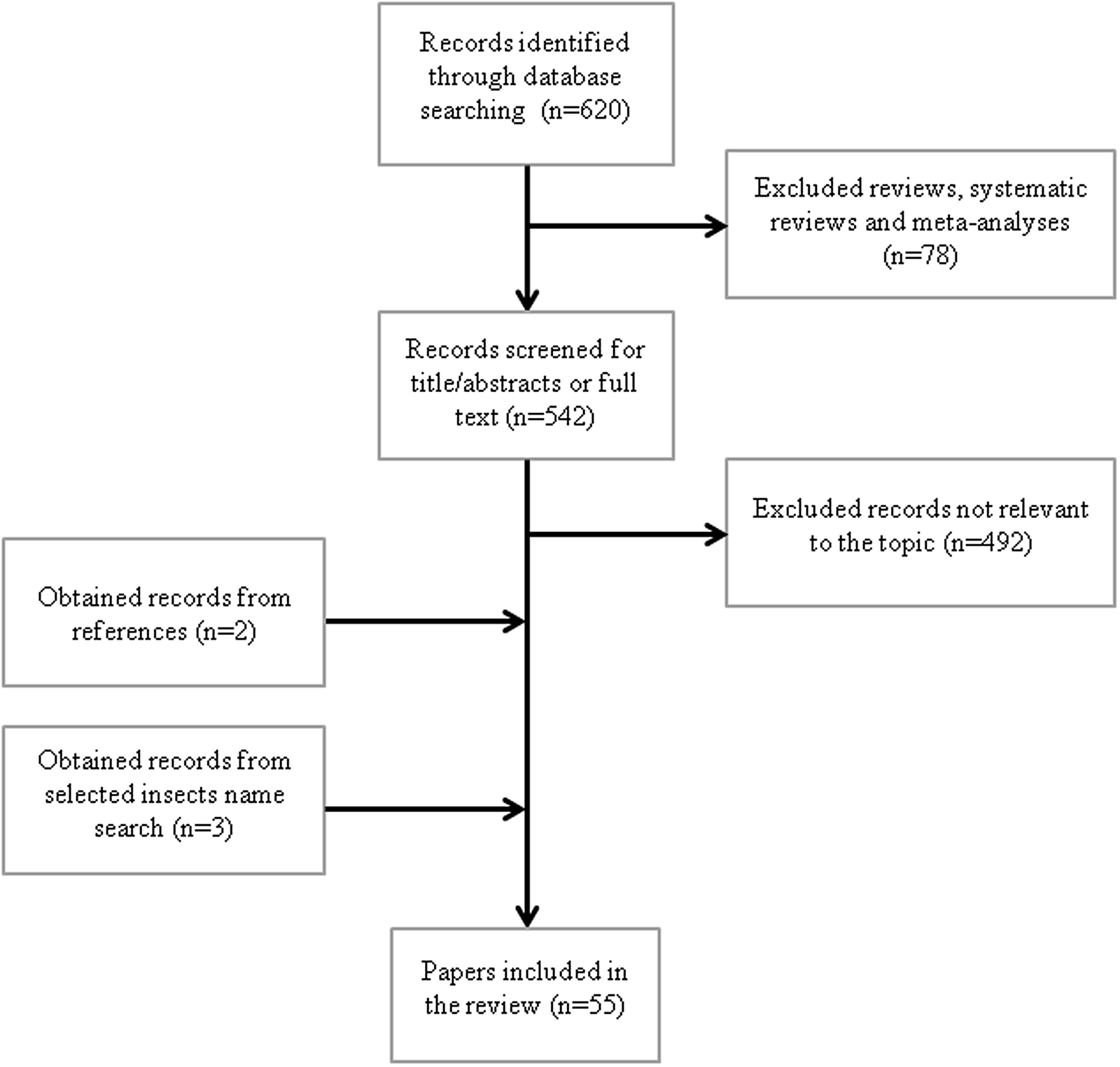
Fig. 1. Flow diagram for search strategy.
Results are presented in Tables 1–6: in vitro studies (Tables 1–3), cellular models/ex vivo studies (Tables 4 and 5) and in vivo (Table 6) studies. They were further grouped by topic: antioxidant activity in vitro (Table 1), in vitro effects on platelet aggregation (Table 2), other in vitro effects (Table 3), antioxidant activity in cellular models or ex vivo (Table 4), and other effects in cellular models/ex vivo (Table 5). For the in vivo studies, results were divided into six categories: body and organ weight and composition, inflammatory status, oxidative/antioxidant status, lipid status, glycaemia/insulin status and coagulation markers.
Table 1. In vitro antioxidant activity of edible insects

PH, protein hydrolysates; DPPH, 2,2-diphenyl-1-picrylhydrazyl; ABTS, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid; FRAP, ferric reducing antioxidant power; ORAC, oxygen radical absorbance capacity; SAHR, scavenging activity on hydroxyl radicals; RSC, superoxide radical scavenging capacity; CAT, catalase; GST, glutathione S-transferase.
Table 2. In vitro anti-platelet aggregation activity of edible insects
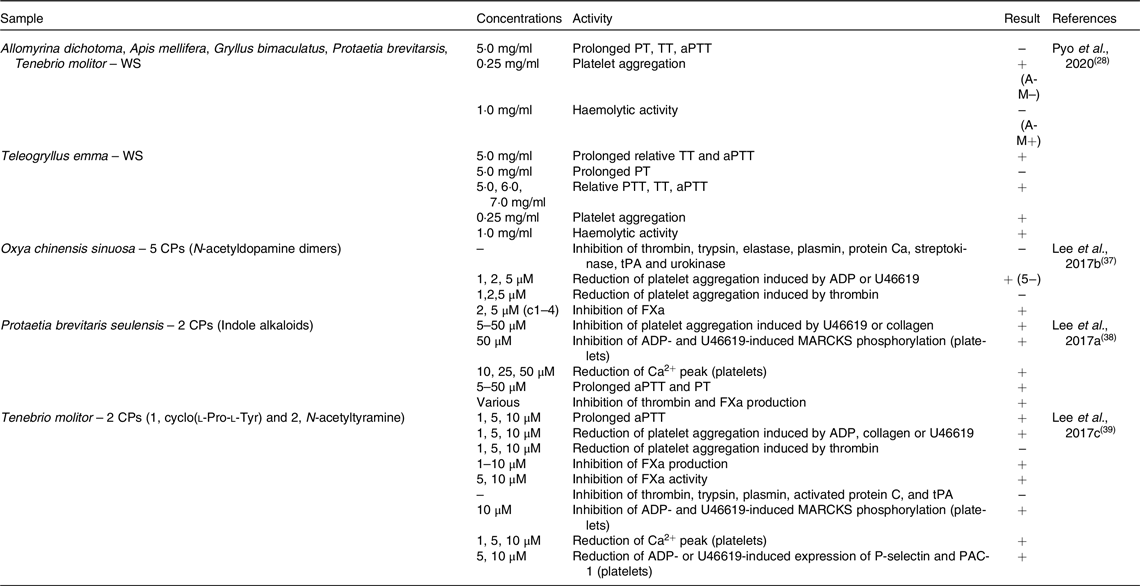
WS, water-soluble extract; PT, prothrombin time; TT, thrombin time; aPTT, activated partial thromboplastin time; CP, compound; tPA, tissue plasminogen activator; ADP, adenosine diphosphate; FXa, factor Xa; MARCKS, myristoylated alanine-rich C-kinase substrate.
Table 3. Other in vitro activity of edible insects

WS, water-soluble extract; PH, protein hydrolysates; ACE, angiotensin converting enzyme; DPP-IV, dipeptidyl peptidase-4; LS, lipo-soluble extract; SCFA, short-chain fatty acids.
Table 4. Antioxidant activity of edible insects in cellular models or ex vivo

PH, protein hydrolysates; NO, nitric oxide; WS, water-soluble extract; MDA, malondialdehyde; ROS, reactive oxygen species; GST, glutathione S-transferase; Nrf2, nuclear factor erythroid 2-related factor; TOS, total oxidant status; TAC, total antioxidant capacity; CP, compound; D-HMVECs, diabetic type 2 microvascular endothelial cells; hPBL, human peripheral blood lymphocytes; HUVECs, human umbilical vein endothelial cells; LS, lipo-soluble extract; CAT, catalase.
Table 5. Other activity in cellular models or ex vivo

HUVECs, human umbilical vein endothelial cells; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; NF-κB, nuclear factor-κB; TNF-α, tumour necrosis factor-α; IL-1β, interleukin-1β; PUFA, polyunsaturated fatty acids; TC, total cholesterol; TBA, total bile acid; LXRα, liver X receptor; PPARγ, peroxisome proliferator-activated receptor γ; ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; CYP7A1, cholesterol 7α hydroxylase; PH, protein hydrolysates; D-HMVECs, diabetic type 2 microvascular endothelial cells; VEGF, vascular endothelial growth factor; WS, water-soluble extract; TG, triacylglycerol; MN, micronucleus; SCE, sister chromatid exchange; CA, chromosome aberration; aPTT, activated partial thromboplastin time; PT, prothrombin time; FXa, factor Xa; PAI-1, plasminogen activator inhibitor-1; tPA, tissue plasminogen activator; C/EBPα, CCAAT/enhancer-binding protein α; FAS, fatty acid synthase; ET-1, endothelin-1; SREBP-1c, sterol regulatory element-binding protein 1c; LPL, lipoprotein lipase; SCD1, stearoyl-CoA desaturase-1; ERK, extracellular signal-regulated kinases; AMPK-α, adenosine monophosphate-activated protein kinase-α; JNK, c-Jun N-terminal kinase.
Table 6. Effect of edible insects on body weight and composition, inflammation, redox, lipid and glycaemia/insulin status and coagulation markers in animal and human studies

CP, compound; LPS, lipopolysaccharide; CLP, caecal ligation and puncture; TG, triacylglycerol; TC, total cholesterol; HDL, high-density lipoprotein; LS, lipo-soluble extract; TAC, total antioxidant capacity; SOD, superoxide dismutase; GPx, glutathione peroxidase; MDA, malondialdehyde; LDL, low-density lipoprotein; WS, water-soluble extract; SCFA, short-chain fatty acids; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; NF-κB, nuclear factor-κB; IL-6, interleukin-6; TNF-α, tumour necrosis factor-α; 8-OHdG, 8-hydroxy-2’ –deoxyguanosine; OGTT, oral glucose tolerance test; AUC, area under curve; ITT, insulin tolerance test; HbA1c, glycosylated haemoglobin concentration; HOMA-IR, homoeostasis model assessment of insulin resistance; ISI, insulin sensitivity index; TLR4, toll-like receptor 4; IL-1β, interleukin-1β; IFN-γ, interferon gamma; IL-4, interleukin-4; ROS, reactive oxygen species; RNS, reactive nitrogen species; GST, glutathione S-transferase; CAT, catalase; IL-10, interleukin-10; F4/80+ KCs, F4/80-positive Kupffer cells; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; ELOVL5, fatty acid elongase 5; ELOVL2, fatty acid elongase 2; FADS2, fatty acid desaturase 2; p-IkB-α, phosphorylated-inhibitor of nuclear factor-kappa B-alpha; GR, glutathione reductase; NEFA, non-esterified fatty acid; ChREBP, carbohydrate-response element-binding protein; SREBP-1c, sterol regulatory element- binding protein 1c; SREBP-2, sterol regulatory element- binding protein 2; CD36, cluster of differentiation 36; FATP5, fatty acid transport protein 5; FABP1, fatty acid-binding protein 1; GPAT1, glycerol-3- phosphate acyltransferase 1; GPAT4, glycerol-3- phosphate acyltransferase 4; AGPAT1, 1-acylglycerol-3-phosphate O-acyltransferase 1; PAP1, phosphatidate phosphatase 1; DGAT1, diacylglycerol O-acyltransferase 1; ADRP, adipose differentiation-related protein; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; ACAT2, acetyl-CoA acetyltransferase 2; SPF, specific pathogen-free; IgE, immunoglobulin E; GI, glucose index; sIgA, secretory immunoglobulin A; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFNα, interferon alpha; IL-1α, interleukin-1 α; IL-2, interleukin-2. IL-5, interleukin-5; IL-7, interleukin-7; IL-8, interleukin-8; IL-12, interleukin-12; IL-13, interleukin-13.
Results
Antioxidant activity in vitro
Table 1 summarises the results of twenty-two studies(Reference Hwang, Chang and Lee6,Reference Messina, Gaglio and Morghese14–Reference Hall, Johnson and Liceaga34) on the in vitro antioxidant activity of different extracts from edible insects. The studies involved thirty-one species of insects, among which the most studied was Tenebrio molitor, tested in ten papers(Reference Messina, Gaglio and Morghese14,Reference Zielińska, Baraniak and Karaś15,Reference Son, Choi and Hwang20–Reference Flores, Casados and Velasco23,Reference Botella-Martínez, Lucas-González and Pérez-Álvarez25,Reference Di Mattia, Battista and Sacchetti26,Reference Pyo, Kang and Jung28,Reference Navarro del Hierro, Gutiérrez-Docio and Otero30) . Acheta domesticus and Gryllodes sigillatus were cited respectively in four and(Reference Messina, Gaglio and Morghese14,Reference Botella-Martínez, Lucas-González and Pérez-Álvarez25,Reference Di Mattia, Battista and Sacchetti26,Reference Navarro del Hierro, Gutiérrez-Docio and Otero30) three papers(Reference Hwang, Chang and Lee6,Reference Zielińska, Baraniak and Karaś15,Reference Hall, Johnson and Liceaga34) , while Alphtobius diaperinus (Reference Di Mattia, Battista and Sacchetti26,Reference Sousa, Borges and Pintado29) , Bombyx mori (Reference Di Mattia, Battista and Sacchetti26,Reference Anuduang, Loo and Jomduang31) , Hermetia illucens (Reference Mintah, He and Dabbour16,Reference Mintah, He and Dabbour17) and Lethocerus indicus (Reference Di Mattia, Battista and Sacchetti26,Reference Shantibala, Lokeshwari and Debaraj27) were investigated in two papers. Water-soluble fractions were investigated in fourteen research articles(Reference Hwang, Chang and Lee6,Reference Alves, Argandoña and Linzmeier19–Reference Tang, Debnath and Choi22,Reference Dutta, Dey and Manna24–Reference Dutta, Dey and Dihingia32) , while protein hydrolysates were reported in six papers(Reference Messina, Gaglio and Morghese14–Reference Mintah, He and Dabbour17,Reference Flores, Casados and Velasco23,Reference Hall, Johnson and Liceaga34) and lipo-soluble fractions were tested in two papers only(Reference Di Mattia, Battista and Sacchetti26,Reference Sun, Xu and Zhang33) . Among the several methods taken into account, the most used was the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method, cited in sixteen studies(Reference Hwang, Chang and Lee6,Reference Messina, Gaglio and Morghese14,Reference Zielińska, Baraniak and Karaś15,Reference Haber, Mishyna and Itzhak Martinez18,Reference Son, Choi and Hwang20–Reference Tang, Debnath and Choi22,Reference Dutta, Dey and Manna24,Reference Botella-Martínez, Lucas-González and Pérez-Álvarez25,Reference Shantibala, Lokeshwari and Debaraj27,Reference Pyo, Kang and Jung28,Reference Navarro del Hierro, Gutiérrez-Docio and Otero30,Reference Dutta, Dey and Dihingia32–Reference Anuduang, Loo and Jomduang35) , while 2,2′-azo-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and ferric reducing antioxidant power (FRAP) methods were performed in thirteen(Reference Hwang, Chang and Lee6,Reference Zielińska, Baraniak and Karaś15,Reference Mintah, He and Dabbour16,Reference Alves, Argandoña and Linzmeier19–Reference Mancini, Fratini and Turchi21,Reference Flores, Casados and Velasco23,Reference Botella-Martínez, Lucas-González and Pérez-Álvarez25,Reference Di Mattia, Battista and Sacchetti26,Reference Pyo, Kang and Jung28,Reference Sousa, Borges and Pintado29,Reference Hall, Johnson and Liceaga34,Reference Anuduang, Loo and Jomduang35) and nine papers(Reference Mintah, He and Dabbour17,Reference Mancini, Fratini and Turchi21,Reference Tang, Debnath and Choi22,Reference Botella-Martínez, Lucas-González and Pérez-Álvarez25,Reference Di Mattia, Battista and Sacchetti26,Reference Pyo, Kang and Jung28,Reference Anuduang, Loo and Jomduang31,Reference Dutta, Dey and Dihingia32,Reference Hall, Johnson and Liceaga34) , respectively. Seven studies investigated the scavenging activity of selected fractions against different radicals(Reference Mintah, He and Dabbour16,Reference Mintah, He and Dabbour17,Reference Tang, Debnath and Choi22,Reference Dutta, Dey and Manna24,Reference Pyo, Kang and Jung28,Reference Sousa, Borges and Pintado29,Reference Dutta, Dey and Dihingia32) , three involving metal ion chelating activity(Reference Zielińska, Baraniak and Karaś15,Reference Botella-Martínez, Lucas-González and Pérez-Álvarez25,Reference Hall, Johnson and Liceaga34) and one utilising β-carotene and linolenic acid bleaching tests(Reference Longvah, Manghtya and Qadri36). All tested fractions showed significant antioxidant activity, the only exception being the protein hydrolysate from Gryllodes sigillatus in FRAP assay(Reference Hall, Johnson and Liceaga34). Only the study conducted by Di Mattia et al. (Reference Di Mattia, Battista and Sacchetti26) provided a comparison between antioxidant activity of water and lipo-soluble fractions of edible insect and food extracts. More specifically, when water-soluble extracts of edible insects were analysed, Calliptamus italicus, Bombyx mori and Acheta domesticus crickets showed an antioxidant capacity, expressed as Trolox equivalent antioxidant capacity (TEAC), five-fold higher than fresh orange juice. Furthermore, water-soluble extracts of Calliptamus italicus, Imbrasia oyemensis and Acheta domesticus displayed a reducing power (FRAP) double that of fresh orange juice. As regards the liposoluble fraction, Bombyx mori, Tanna japonensis and Imbrasia oyemensis showed a TEAC twice that of olive oil. Regarding endogenous antioxidants, Dutta et al. (2016) showed that an aqueous extract of Vespa affinis was able to increase the activity of catalase (CAT) and glutathione S-transferase (GST)(Reference Dutta, Dey and Manna24).
Anti-platelet aggregation activity in vitro
Seven edible insects were tested for their effects on coagulation markers as described in Table 2. In the study by Pyo and colleagues (2020)(Reference Pyo, Kang and Jung28), ethanol extract of the edible insect Teleogryllus emma was able to prolong thrombin time (TT) and activated partial thromboplastin time (aPTT), but not prothrombin time (PT); also relative PT, TT and aPTT were increased. In the same study, Allomyrina dichotoma, Apis mellifera, Gryllus bimaculatus, Protaetia brevitarsis and Tenebrio molitor did not exert the same effect. However, all above-mentioned insects, with the exception of Apis mellifera, were able to increase platelet aggregation; conversely, haemolytic activity was observed only in Apis mellifera and Teleogryllus emma. N-acetyl dopamine dimers obtained from Oxya chinensis sinuosa showed the ability of inhibit factor Xa (FXa) and to reduce platelet aggregation induced by adenosine diphosphate (ADP) or U46619, but not by thrombin. However, they did not inhibit thrombin, trypsin, elastase, plasmin, streptokinase, tissue plasminogen activator (tPA) or urokinase(Reference Lee, Lee and Kim37). Indole alkaloids isolated from Protaetia brevitaris seulensis affected coagulation by inhibiting platelet aggregation, myristoylated alanine-rich C-kinase substrate (MARCKS) phosphorylation and reduced Ca2+ peak in platelets. Moreover, they were able to prolong aPTT and PT and to inhibit thrombin and FXa production(Reference Lee, Lee and Kim38). Finally, two compounds (1, cyclo(l-Pro-l-Tyr) and 2, N-acetyltyramine) extracted from Tenebrio molitor prolonged aPTT and reduced platelet aggregation induced by ADP, collagen or U46619, but not by thrombin. Moreover, FXa production and activity were inhibited, but not thrombin, trypsin, plasmin, activated protein C or tPA. These compounds also inhibited MARCKS phosphorylation and expression of P-selectin and PAC-1, and reduced Ca2+ peak in platelets(Reference Lee, Kim and Park39).
Other activities in vitro
Table 3 presents seven studies(Reference Alves, Argandoña and Linzmeier19,Reference Sousa, Borges and Pintado29,Reference Navarro del Hierro, Gutiérrez-Docio and Otero30,Reference Hall, Johnson and Liceaga34,Reference Yoon, Wong and Chae40–Reference De Carvalho, Teixeira and Silva42) evaluating other in vitro effects for seven different insects. Proteins from Bombyx mori, Tenebrio molitor and Gryllus bimaculatus (Reference Yoon, Wong and Chae40), Alphitobius diaperinus (Reference Sousa, Borges and Pintado29) and Gryllodes sigillatus (Reference Hall, Johnson and Liceaga34,Reference Hall, Reddivari and Liceaga41) were able to inhibit activity of angiotensin-converting enzyme (ACE). These proteins also showed potential anti-diabetic activity by inhibition of dipeptidyl peptidase-4 (DPP-IV)(Reference Hall, Johnson and Liceaga34) and α-glucosidase(Reference Yoon, Wong and Chae40,Reference Hall, Reddivari and Liceaga41) . Moreover, extracts from Acheta domesticus and Tenebrio molitor were able to inhibit pancreatic lipase(Reference Navarro del Hierro, Gutiérrez-Docio and Otero30), while Pachymerus nucleorum larvae showed tryptic activity together with absence of anti-nutritional factors as well as anti-tryptic and chymotryptic activity(Reference Alves, Argandoña and Linzmeier19). Lastly, flour from Tenebrio molitor affected the growth of Lactobacillus and Bifidobacterium, improving short-chain fatty acid (SCFA) production and viability in nutritive stress conditions(Reference De Carvalho, Teixeira and Silva42).
Antioxidant activity in cellular models
Nine studies(Reference Hwang, Chang and Lee6,Reference Son, Choi and Hwang20,Reference Dutta, Dey and Manna24,Reference Dutta, Dey and Dihingia32,Reference Lee, Kim and Park39,Reference Yoon, Wong and Chae40,Reference Koc, Incekara and Turkez43–Reference Ahn, Kim and Kim45) investigating the antioxidant activity in cellular model of ten species of insects are described in Table 4. Gryllus bimaculatus and Tenebrio molitor were the most utilised insects, examined in three studies. Different fractions of the insects, including water-soluble fraction (n = 6), compounds (n = 2), protein hydrolysates (n = 1) and lipo-soluble fraction (n = 1), were tested. In three different studies, aqueous extracts of Gryllus bimaculatus (Reference Hwang, Chang and Lee6), methanolic extract of defatted powder and unsaponifiable lipids, obtained by Tenebrio molitor (Reference Son, Choi and Hwang20), and Bombyx mori protein hydrolysates showed the ability to reduce lipopolysaccharide-induced nitric oxide (NO) production in the murine macrophage cell line RAW 264·7. A similar effect was exerted by 1, cyclo(L-Pro-L-Tyr) and 2, N-acetyltyramine isolated from Tenebrio molitor in human umbilical vein endothelial cells (HUVECs)(Reference Lee, Kim and Park39) and by glycosaminoglycan from Gryllus bimaculatus in diabetic type 2 microvascular endothelial cells (D-HMVECs)(Reference Ahn, Kim and Kim45). According to a study by Yoon and co-workers(Reference Yoon, Wong and Chae40), protein hydrolysates of Tenebrio molitor and Gryllus bimaculatus did not exert any effect on NO release. Water-soluble extract of dung beetles of Onitis sp., mole crickets of Gryllotalpa sp., grasshopper of Caelifera sp.(Reference Koc, Incekara and Turkez43), Oryctes boas and Zonocerus variegatus (Reference Memiş, Türkez and İncekara44) were tested in human peripheral blood lymphocytes to evaluate whether they affected oxidative status. Results showed that at lower concentrations (10–40 ppm) the insects display an antioxidant effect; however, at higher concentrations (2000 ppm) they exhibited a pro-oxidant effect. According to the results obtained in a cell-free system(Reference Dutta, Dey and Manna24), the aqueous extract of Vespa affinis was able to increase the activity of both GST and CAT also in THP-1 human monocytes and human plasma; moreover, it reduced reactive oxygen species (ROS) formation in THP-1. Finally, the hydro-alcoholic extract of Brachytrupes oriental was able to restore nuclear factor erythroid 2-related factor (Nrf2) and GST protein expression, reducing radical and malondialdehyde (MDA) levels in C2C12, a murine myotube cell line, following high glucose stress(Reference Dutta, Dey and Dihingia32).
Other activity in cellular models or ex vivo
The effect of edible insects on coagulation markers(Reference Lee, Lee and Kim37–Reference Lee, Kim and Park39), inflammatory status(Reference Hall, Reddivari and Liceaga41,Reference Park, Lee and Yoo46) and lipid metabolism(Reference Luo, Wang and Lv7,Reference Im, Yang and Park47–Reference Ahn, Myung and Jung49) are described in Table 5. A total of eleven studies involving eight insects were reported: Protaetia brevitaris was cited in three research articles, whilst Oxya chinensis, Gryllus bimaculatus and Tenebrio molitor were investigated in two studies each. Compounds (indole alkaloids) isolated from Protaetia brevitaris seulensis were able to reduce prothrombin-produced thrombin, plasminogen activator inhibitor (PAI-1) and FXa, but not tPA, in HUVECs. Moreover, in platelet-rich plasma (ex vivo) from mouse, they were able to reduce aggregation percentage and increase aPTT and PT(Reference Lee, Lee and Kim38). Compounds (N-acetyl dopamine dimers) extracted from Oxya chinensis sinuosa were able to increase aPTT but did not affect PT in mice plasma (ex vivo); in the same study, they also showed the ability to reduce FXa in HUVECs(Reference Lee, Lee and Kim37). Lastly, two compounds (1, cyclo(l-Pro-l-Tyr) and 2, N-acetyltyramine) from Tenebrio molitor were able to increase aPPT and clotting time in mice blood (ex vivo) and increase endothelin-1 (ET-1) production – a vasoconstrictor – in HUVECs(Reference Lee, Kim and Park39).
In four studies(Reference Luo, Wang and Lv7,Reference Im, Yang and Park47–Reference Ahn, Myung and Jung49) , the ability of edible insects to modulate lipid pattern in cellular models was investigated through evaluation of lipid content or the expression of genes related to lipid metabolism. Ethanolic extract of Gryllus bimaculatus, Oxya chinensis sinuosa and Protaetia brevitarsis seulensis diminished intracellular lipid accumulation and triacylglycerol (TG) in HepG2, a human liver cancer cellular line(Reference Im, Yang and Park47). Moreover, lipid accumulation, together with total cholesterol (TC) levels, was reduced in L-02 cells, a human fetal hepatocyte line, by polyunsaturated fatty acids (PUFAs) and α-linolenic acid from Bombyx mori. As reported in the same paper, the extracts showed the ability to increase the mRNA and protein expression of cholesterol metabolism-related genes(Reference Luo, Wang and Lv7). Incubation with Tenebrio molitor larvae decreased lipid droplet formation and TG levels in 3T3-L1 cells (murine pre-adipocytes) and mRNA expression of genes related to adipogenic differentiation and lipogenesis. Additionally, the treatment decreased phosphorylation of extracellular signal-regulated kinase (ERK) and increased phosphorylation of adenosine monophosphate-activated protein kinase-α (AMPK-α) and p-p38 but did not affect phosphorylation of c-Jun N-terminal kinase (JNK)(Reference Seo, Goo and Chung48). In the same cellular model, ethanolic extract of Protaetia brevitaris larvae decreased the mRNA expression of genes related to adipogenesis(Reference Ahn, Myung and Jung49).
Two studies investigated the effect of edible insects on inflammatory response in cellular lines. In particular, Park and colleagues(Reference Park, Lee and Yoo46) evaluated how three tetrahydroquinolines from Allomyrna dichotoma affected vascular inflammatory responses in HUVECs: they reduced vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) levels, adherence of monocytes to HUVECs monolayers and migration of human neutrophils; moreover, they decreased nuclear factor-κB (NF-κB) p65 activity, tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) production and expression of phospho-p38. Furthermore, protein hydrolysates from Gryllodes sigillatus reduced NF-κB in RAW 264·7 cells(Reference Hall, Reddivari and Liceaga41). Aqueous extract of Locusta migratoria, tested on human peripheral blood lymphocytes, did not affect the ratio between micronucleus (MN) and cells, sister chromatid exchange (SCE) and chromosome aberration (CA) formation(Reference Turkez, İncekara and Güner50). Finally, in D-HMVECsm glycosaminoglycan from Gryllus bimaculatus increased vascular endothelial growth factor (VEGF), but not laminin(Reference Ahn, Kim and Kim45).
Effect of edible insects in animal and human studies
A total of twenty-five intervention studies in animal models and two in humans have been published, as described in Table 6. Fifteen different edible insects were investigated: five papers involved Gryllus bimaculatus (Reference Hwang, Chang and Lee6,Reference Ahn, Kim and Kim45,Reference Im, Yang and Park47,Reference Ahn, Kim and Kwon51,Reference Karna, Choi and Kim52) and four Tenebrio molitor (Reference Lee, Kim and Park39,Reference Seo, Goo and Chung48,Reference Choi, Ham and Ryu53,Reference Choi, Ji and Lee54) , while the use of Oxya chinensis sinuosa (Reference Lee, Lee and Kim37,Reference Im, Yang and Park47,Reference Im, Park and Ji55) and Protaetia brevitaris seulensis (Reference Lee, Lee and Kim38,Reference Im, Yang and Park47,Reference Ahn, Myung and Jung49) was reported in three interventions each. One study investigated the effects of both acute and chronic settings, while four were only acute (i.e. single dose) and twenty were chronic (medium to long term) interventions. Six studies(Reference Longvah, Manghtya and Qadri36,Reference Ryu56–Reference Kim, Kwak and Park60) were conducted on animals that were not affected by particular stresses. Among these, Kim and colleagues investigated any toxic effects of a skimmed powder obtained from Zophobas atratus on specific pathogen-free (SPF) Sprague-Dawley rats for a 2-week repeated-dose toxicity study. Neither toxicological lesions nor mortality was observed, nor was body and organ weight influenced. The same outcomes were observed in a 13-week repeated-dose toxicity study; moreover, in macroscopic or histopathological examinations, no test-substance-related toxicological lesions were observed(Reference Kim, Kwak and Park60). No toxicity signs were observed in healthy Wistar rats after the acute ingestion of locust (Caelifera sp.) powder, which was therefore administered to the same animal model for 28 or 90 d; organ and tissue weight, as well as TG, TC and high-density lipoprotein (HDL) cholesterol plasma levels, were not affected. However, TG and TC liver contents were increased after 90 d of supplementation, as faecal crude lipids. As regards caecum content, TG and TC concentration did not change, while SCFA and insulin levels were decreased(Reference Ochiai, Inada and Horiguchi57). The oil extracted from Samia ricinii was administered to healthy weanling Wistar National Institute of Nutrition (NIN) rats for 18 weeks, causing no variation in body weight gain or organ weight. Serum levels of TG and TC were reduced, while low-density lipoprotein (LDL) level was increased; TG content of liver was not affected(Reference Longvah, Manghtya and Qadri36). Effects on lipid status were observed also in a study by Ryu(Reference Ryu56): 5 weeks of a diet comprising powder of whole Bombyx mori did not affect their body weight or HDL levels, but reduced TG and TC, of healthy Sprague-Dawley rats(Reference Ryu56). Neither body weight gain nor liver and kidney weight were altered in healthy Sprague-Dawley rats after 4 weeks of supplementation with grain larvae. However, their thymus and spleen weight increased, while abdominal fat was reduced. A reduced level of immunoglobulins (IgA, IgM, IgG), blood glucose and TG, TC and LDL was reported. Furthermore, this treatment modified the composition of caecal organic acids, increasing their total quantity and the acetic and propionic acid level, whilst reducing butyric, isobutyric, valeric and isovaleric acid contents(Reference Park and Park58).
Zebrafish fed with diet containing different percentages of Hermetia illucens (75–100 % of total meal) showed a significant increase in the specific growth rate when compared with the control, while survival was not affected. Considering mRNA expression of IL-1β, interleukin 10 (IL-10) and TNF-α, groups fed with this percentages of insect powder showed a significant up-regulation with respect to control; furthermore, this condition induced a severe degree of steatosis. As concerns lipid metabolism-related gene expression, no significant differences in fatty acid elongase 2 (ELOVL2) or fatty acid desaturase 2 (FADS2) were detected, while fatty acid elongase 5 (ELOVL5) was up-regulated. Conversely, lower doses (25–50 %) did not significantly affect the previously reported parameters. However, all the doses were able to increase the percentage of lipid categories such as saturated fatty acids (SFA) and n-6 and to decrease monounsaturated fatty acids (MUFA), PUFA, n-3 and n-9 in zebrafish; histological analyses of fish intestine did not show any morphological alteration or inflammatory event(Reference Zarantoniello, Randazzo and Gioacchini59).
Two studies(Reference Agbemafle, Hanson and Bries61,Reference Bergmans, Nikodemova and Stull62) were conducted on malnourished animals. In particular, powder of whole Acheta domesticus or Rhynchophorus phoenicis fabricius was included in the diet of malnourished weanling Sprague-Dawley rats, increasing their body weight gain, organ (spleen, right kidney, brain, liver) weight, bone mineral content and lean mass. Fat mass was increased only by Acheta domesticus; moreover, the relative biological value of the diet was improved by the insect addition(Reference Agbemafle, Hanson and Bries61). Six weeks of a diet based on Gryllodes sigillatus helped malnourished mice to recover by increasing their body weight; compared with the control group, whose mice did not suffer from malnutrition, the treated mice did not show changes in levels of several inflammatory markers, such as toll-like receptor 4 (TLR4), TNF-α, IL-1β and interferon γ (IFN-γ), and anti-inflammatory markers, such as interleukin 4 (IL-4), in spleen tissue. Similarly, leptin and adiponectin levels were not affected, while triacylglycerols were reduced(Reference Bergmans, Nikodemova and Stull62).
The effect of edible insect consumption on animals fed a high-fat diet was evaluated in six studies(Reference Im, Yang and Park47–Reference Ahn, Myung and Jung49,Reference Ahn, Kim and Kwon51,Reference Xia, Chen and Wu63,Reference Zou, Hu and Shi64) . Chitooligosaccharides from Clanis bilineata administered for 6 weeks improved the lipid status of Sprague-Dawley rats fed a high-fat diet by reducing TC, TG and LDL and increasing HDL levels. Faecal excretion of TG and TC was increased, while food efficiency ratio was decreased; although food intake remained stable, body weight gain decreased(Reference Xia, Chen and Wu63).
The ethanolic extracts of Protaetia brevitaris larvae, administered with a high-fat diet for 7 weeks, decreased body weight gain, epididymal and subcutaneous fat weight and liver – but not spleen – weight of obese C57BL/6J mice; the treatment increased glutathione peroxidase (GPx) and CAT and reduced lipid droplet accumulation in mouse liver, as well as TG, TC and LDL levels(Reference Ahn, Myung and Jung49). Ethanolic extract of Gryllus bimaculatus, added to a high-fat diet, did not affect body weight or epididymal fat in obese rats, but it reduced total and abdominal fat weight. Moreover, it changed the composition of abdominal fat, reducing MUFA and increasing PUFA content. The supplementation did not affect TC, HDL or LDL levels, but reduced TG, IL-10 and glucose levels. Even though serum CAT was not affected, the prolonged treatment with ethanolic extract of Gryllus bimaculatus reduced protein and lipid oxidative damage caused by high-fat diet in both liver and blood, where serum uric acid and carbonyl concentrations were reduced(Reference Ahn, Kim and Kwon51).
In a study by Im and colleagues(Reference Im, Yang and Park47), the ethanol extracts of three different insects, that is, Gryllus bimaculatus, Oxya chinensis sinuosa and Protaetia brevitaris seulensis, were administered for 14 weeks to C57BL/6J mice subjected to a high-fat diet to counteract the effects of non-alcoholic fatty liver disease. All the three supplementations reduced body weight gain and liver weight, together with intestinal and epididymal adipose tissue, but no significant effect was described for abdominal and kidney adipose tissue. Moreover, when inflammation markers were measured in liver and adipose tissue, mRNA levels of TNF-α, IL-1β and interleukin 6 (IL-6) were found to be decreased. Oxya chinensis sinuosa and Gryllus bimaculatus reduced lipid droplet accumulation in liver, TG, TC, LDL, HDL and non-esterified fatty acid (NEFA) levels, together with fasting blood glucose, while among these markers Protaetia brevitaris seulensis reduced only lipid droplet accumulation, TC, NEFA and fasting blood glucose(Reference Im, Yang and Park47).
Zou et al. (Reference Zou, Hu and Shi64) reported that, in Wistar rats with hypercholesterolaemia, Bombyx mori pupae oil supplementation was able to counteract the impairments induced by a high-cholesterol diet: lipid status was improved through a reduction of TC, LDL and LDL/HDL ratio without affecting TG or HDL levels. Moreover, the size of liver, kidney and heart was reduced, while that of spleen was increased. The supplementation improved antioxidant status, restoring superoxide dismutase (SOD) levels, increasing TAC levels, decreasing MDA in liver and serum and restoring the activity of GPx in rats’ liver(Reference Zou, Hu and Shi64). Finally, 6 weeks of treatment of obese BALB/c mice with whole powder of Tenebrio molitor resulted in a decrease in body weight gain and epididymal and abdominal-to-peripheral adipose cell volume, as well as a decrease in lipid accumulation in liver(Reference Seo, Goo and Chung48).
Two studies(Reference Zhao, Wang and Wei8,Reference Ahn, Kim and Kim45) investigated whether the consumption of edible insects was able to counteract the effect of diabetes in mice. The supplementation with ethanol extract of the sericin layer from the green cocoon shell of Bombyx mori improved glycaemic status in obese mice with type 2 diabetes, increasing blood glucose reduction rate and reducing blood glucose during tolerance tests, fasting blood glucose and insulin, glycosylated haemoglobin (HbA1c) and homoeostasis model assessment of insulin resistance (HOMA-IR), while improving insulin sensitivity index (ISI). Pancreas functionality was restored: islet structure and area of pancreatic beta cells and the insulin quantity secreted by them was increased. Treatment also improved antioxidant status, increasing the activity of liver GPx and SOD and reducing the liver content of MDA and 8-hydroxy-2’-deoxyguanosine (8-OHdG). Inflammation markers in liver, as well as infiltrations and oedema and NF-kB, IL-6 and TNF-α levels, were also reduced, while body weight was not affected(Reference Zhao, Wang and Wei8). Glycosaminoglycan extracted from Gryllus bimaculatus and administered for 1 month to BKS.Cg-m+/+Leprdb diabetic mice, despite not affecting body weight and abdominal fat, reduced circulating levels of carbonyl. As regards antioxidant enzymes, this extract did not affect GST, but improved activity of CAT and GPx. It also reduced adipocyte density in lungs, liver and kidneys, but not in pancreas. However, the treatment improved the status of pancreas islet and liver tissue that was damaged by the diabetic condition. Blood glucose levels were not affected, whilst non-fasting blood glucose was reduced only after 1 week of treatment(Reference Ahn, Kim and Kim45).
The effect of edible insects against stress induced by alcohol consumption was evaluated in three different studies(Reference Hwang, Chang and Lee6,Reference Choi, Ham and Ryu53,Reference Choi, Ji and Lee54) . The first reported positive action of the aqueous extract of Gryllus bimaculatus in restoring the normal physiological levels of 8-OHdG levels and MDA content in liver and small intestine of C57BL/6J mice with liver damage caused by acute alcohol exposure; liver droplet accumulation was also reduced, as well as levels of inflammation markers (F4/80-positive Kupffer cells (F4/80+ KCs) and IL-1β)(Reference Hwang, Chang and Lee6). The treatment with fermented Tenebrio molitor powder of Sprague-Dawley rats, impaired by a chronic alcohol diet, reduced liver weight and increased activity of glutathione reductase (GR) and total and reduced GPx hepatic content, but no effect was observed on SOD and CAT levels. Moreover, phosphorylated-inhibitor of nuclear factor-kappa B-alpha (p-IκB-α) levels were reduced, as well as serum and liver content of TNF-α, but not of IL-6. As regards lipid status, hepatic levels of TG, NEFA and TC were also reduced, as alcohol-induced hepatic lipid accumulation, focal necrosis and mild fibrosis were attenuated. Also, expression of lipid synthesis transcription factors, triacylglycerol synthesis-related genes and cholesterol synthesis and esterification-related genes was reduced, while expression of fatty acid uptake and transport-related genes was not affected.(Reference Choi, Ham and Ryu53). The treatment with fermented defatted Tenebrio molitor powder of Sprague-Dawley rats fed with a chronic alcohol diet dose-dependently increased hepatic β-oxidation(Reference Choi, Ji and Lee54).
Concerning the effect on coagulation markers(Reference Lee, Lee and Kim37–Reference Lee, Kim and Park39), compounds extracted from Protaetia brevitaris seulensis (Reference Lee, Lee and Kim38), Oxya chinensis sinuosa (Reference Lee, Lee and Kim37) and Tenebrio molitor (Reference Lee, Kim and Park39) decreased arterial thrombi formation rate and size in mice with arterial thrombosis and decreased mortality in mice with pulmonary thrombosis; in this model, 1, cyclo(l-Pro-l-Tyr) and 2, N-acetyltyramine from Tenebrio molitor were also able to reduce thrombi size(Reference Lee, Kim and Park39). Indole alkaloids and N-acetyldopamine dimers extracted respectively from Protaetia brevitaris seulensis (Reference Lee, Lee and Kim38) and Oxya chinensis sinuosa (Reference Lee, Lee and Kim37) increased tail bleeding time in healthy mice.
Edible insects were also able to improve antioxidant and inflammatory status that was previously impaired by different stressing agents such as sepsis(Reference Park, Lee and Yoo46), varicocele(Reference Karna, Choi and Kim52) or UV radiation(Reference Im, Park and Ji55). Four tetrahydroquinolines from Allomyrina dichotoma were administered twice to caecal ligation and puncture (CLP)-induced septic C57BL/6 mice, resulting in an increase in the survival rate. There were no significant differences between the lungs of the treated and untreated mice: interstitial oedema was observed, and the pulmonary architecture was severely damaged. Moreover, to evaluate effect of compounds against inflammation, lipopolysaccharide (LPS)-induced vascular permeability in mice was inhibited by the tetrahydroquinolines(1–Reference Ercolini and Fogliano3), which reduced LPS-induced leucocyte migration into the murine peritoneal cavities(Reference Park, Lee and Yoo46). Moreover, treatment with Gryllus bimaculatus improved antioxidant and anti-inflammatory status in testicular tissue of Sprague-Dawley rats affected by varicocele. Furthermore, the increased levels of MDA, ROS and reactive nitrogen species (RNS) were significantly reduced(Reference Karna, Choi and Kim52). Finally, the extract of Oxya chinensis sinuosa, administered to Hos/HR-1 hairless mice for 12 weeks, reduced the damage induced by UV irradiation as well as inflammation markers (IL-1-β, IL-6 and TNF-α), while the activity of SOD and CAT was increased(Reference Im, Park and Ji55).
Evidence in humans is available only for two dietary intervention trials. Wheat noodles enriched with Bombyx mori powder, were provided to thirteen healthy humans, fasting in the morning, following a cross-over acute ingestion design. The Bombyx mori noodles significantly reduced post-prandial blood glucose, glucose peak, area under the curve (AUC) of glucose and glucose index (GI) compared with control noodles(Reference Suk, Kim and Kim65). In a recent chronic intervention study, 25 g/d of dried roasted cricket powder, included in a muffin and in a dry mix shake, was given to twenty healthy humans for breakfast for 14 d following a cross-over design. The treatment did not modify intestinal microbiota, gastrointestinal functionality, glycaemia or IgA levels of the subjects. However, TNF-α plasma levels decreased, as did the acetate, propionate, bile acids and TG content of faeces(Reference Stull, Finer and Bergmans66).
Discussion
In this work, we summarised the body of evidence on selected functional properties of edible insects in modulating oxidative and inflammatory stress, platelet aggregation, lipid and glucose metabolism and weight control in different experimental models. Concerning the considered species, among a total of forty-three edible insects, the Gryllidae family and Tenebrio molitor were the most investigated, respectively in seventeen and sixteen studies. Both species are included in the list of insects for human consumption from the European Food Safety Authority and are also widely present in the market. Regarding the different aspects, antioxidant properties were investigated in thirty-six different species (Tenebrio molitor and Gryllidae in fourteen and twelve studies, respectively); eleven species were tested for their ability to affect lipid status (Gryllus bimaculatus and Bombyx mori in four studies and Tenebrio molitor in three studies). As regards the anti-inflammatory properties, the effects of nine insects were investigated: Gryllus bimaculatus was cited in five research articles, whilst Grillodes sigillatus and Bombyx mori were tested in two studies each. Gryllus bimaculatus was investigated for its ability to modulate glucose metabolism in four different studies, followed by Gryllodes sigillatus with two studies. Lastly, the studies involving coagulation markers investigated seven insects, of which Tenebrio molitor and Protaetia brevitaris seulensis were evaluated twice each. It is noticeable that the Gryllidae family, represented mainly by Gryllus bimaculatus and Gryllodes sigillatus, is the most cited in all the topics, the only exception being the effect on coagulation. Moreover, one of the two studies on human subjects involved crickets of the Gryllidae family, while the other study was focused on Bombyx mori.
The antioxidant properties of edible insects were investigated in thirty-six out of fifty-five studies involving animals (n = 11), cell cultures (n = 9) or in vitro (n = 22). Results clearly show that all insects tested in vitro and in cellular models displayed radical scavenging or metal ion chelation properties, as well as the ability to modulate glutathione S-transferase and catalase, with activity depending on the utilised concentration. Also, the findings in animal models were consistent with in vitro results, supporting the antioxidant properties of edible insects. According to the different studies, the effect was evident in serum, liver and skin, with a mechanism of action ranging from the increase in antioxidant capacity up to the modulation of endogenous antioxidant enzymes. In the majority of the studies, the antioxidant effect was more evident when specific stressors such as high-fat diet, oxidative stress, obesity, alcoholic liver disease or UV irradiation were present. Although the antioxidant properties have been widely investigated in vitro and in cellular and animal models, studies on human subjects are lacking. Overall, the evidence from the available studies clearly showed that all the tested insects, with varying ability, were able to reduce an induced oxidative stress, modulating redox status of cellular and body fluids and restoring the impaired activity of antioxidant enzymes.
The effect on lipid markers was evaluated in twenty-one research articles; the studies were carried out mostly in animal models (n = 18), while only few were in cellular models (n = 4) or in vitro (n = 1). When markers of dyslipidaemia were evaluated in animal studies, edible insects were able to reduce TG (10/12), TC (7/11) and LDL (6/8) levels, without affecting HDL (5/7), in body fluids and tissues. Moreover, the reduction of TG was detected also in hepatocytes and adipocytes, while a study reported a reduction of TC in hepatocytes. Reduction of lipid droplets in liver was detected in animal models (7/7) and in two studies on cellular model of hepatocytes, suggesting an effect on liver steatosis. A reduction in fat tissue weight or volume was detected in five out of seven studies, together with a change in fat composition (2/2). Furthermore, edible insects positively modulated lipid metabolism and fat accumulation in animal (n = 2) and cellular models (n = 3). The only in vitro study evaluating the ability of edible insects to affect lipid metabolism reported an inhibition of pancreatic lipase, with the possible consequence of preventing the breakdown of triacylglycerol and delaying the absorption of fatty acids(Reference Rajan, Palaniswamy and Mohankumar67). Furthermore, the effect of consumption of edible insects on weight control, expressed as body weight (BW) or body weight gain (BWG) was evaluated in fifteen studies; considering both parameters, either an increase (2/15) or a decrease (4/15) was reported, but in most of the cases no significative effect was observed (9/15). However, if the results are classified on the basis of the type of diet or the body weight at the baseline, the supplementation with edible insects induced a decrease in BW or BWG in four out of five cases in obese animals or following a high-fat diet, while an increase of BW was reported when malnourished animals were considered (n = 2). Based on the evidence from animal studies, dietary intervention with edible insects reduced TC, TG and LDL, while the effect on BW was dependent on whether animals were obese or malnourished.
The anti-inflammatory properties of edible insects were evaluated in sixteen papers, twelve in vivo and five in cellular models. Concentrations of cytokines were evaluated in nine in vivo studies, leading to a reduction in circulating levels increased by different stressors (8/9). An increase in cytokine levels was observed only when high doses of Hermetia illucens (75–100 % of the total diet) were given to healthy zebrafish(Reference Zarantoniello, Randazzo and Gioacchini59), although no inflammatory events were observed through histological analysis. An effect on reducing circulating levels of TNF-α was shown in humans, although we need to consider that the study was conducted on healthy subjects, who may have low or scarce levels of inflammation, and that the number of subjects enrolled was low(Reference Stull, Finer and Bergmans66). Effects on immunoglobulins were evaluated in two papers: IgE levels – as an identifier of allergic reactions in rats – were not detected, whilst an increase in IgG, IgA and IgM was revealed, considered by the authors to be a bifidogenic effect from the antimicrobial peptides in the used extract. NF-κB levels, transcription-factor-regulating genes involved in inflammatory responses, were decreased in cellular models, HUVECs and RAW 264·7, and in an animal study. However, TLR4 levels, whose stimulation leads to NF-κB activation, were not affected. Concerning the effect on specific organs, reduction of inflammatory infiltrations and oedema in liver, leucocyte migration and vascular permeability in peritoneum were observed. Finally, three studies showed activity in reducing NO production in macrophages, a radical involved in the modulation of inflammation and immunity(Reference Lee, Rey and Besler68). Therefore, the evidence from cellular and animal models supports an effect on reducing inflammatory cytokines thorough the modulation of NF-κB levels, without affecting immunoglobulins.
Effects of edible insects on glucose and/or insulin status were evaluated in nine studies; six out of the total were in vivo interventions, and three were in vitro studies. Most of the in vivo studies reported improvements in diabetes markers, such as reduced blood glucose (4/6) and serum insulin (2/2), as well as an enhancement of pancreas structure and functionality (2/2). Moreover, a reduction in glycated haemoglobin (Hba1c), a tool in both routine management and diagnosis of diabetes(Reference Weykamp69), was detected. However, only two interventions out of six involved diabetic mice. All the in vitro studies outlined a positive effect through the inhibition of DPP-IV and α-glucosidase, suggesting a role in modulating glucose homoeostasis(Reference De, Banerjee and Kumar70). The results in vitro and in animal models are supported by evidence in humans showing an effect of noodles enriched with Bombyx mori powder on reducing glycaemic index (Reference Suk, Kim and Kim65). Although further evidence is needed, results suggest that edible insects can modulate glycaemia/insulin homoeostasis with potential effect on the management of glycaemic index, a key aspect for diabetes prevention.
Anti-coagulation properties of edible insects have been investigated in only four different works: three out of four studies were performed by the same research group, investigating compounds extracted from one insect for each, using either in vitro, ex vivo, cellular or animal models vivo with acute interventions, while the other study investigated in vitro the properties of seven different insects. The selected edible insects showed anti-coagulant properties in vitro and ex vivo, by reducing aPTT and PT – both measuring speed of blood clotting – and affecting the coagulation cascade by modulation of factor X and thrombin. Moreover, a reduction in PAI-1 was recorded, whose inhibition may reduce the incidence of thrombotic events(Reference Gils and Declerck71). PAI-1 is also an important physiological inhibitor of tPA(Reference Pant, Kopec and Baker72): this molecule, whose main function within the vascular system is the removal of fibrin(Reference Kruithof and Dunoyer-Geindre73), was not affected by in vitro treatment with compounds extracted from edible insects. When compounds derived from edible insects were administered to mice in acute interventions, a decrease of thrombi formation and consequent mortality in mice models of arterial or pulmonary thrombosis was observed; moreover, these compounds prolonged tail bleeding time in healthy mice. Overall, the available literature is scarce and from one research group only, making it hard to draw any conclusion on the anti-platelet aggregation properties of edible insects.
In terms of research quality of the results, the evidence from in vitro, cellular and animal studies is solid because the markers and biomarkers utilised are appropriate and the animal studies are properly designed. Human studies involved a low number of subjects, short period of dietary intervention and lack of a dietary assessment of the subjects during the studies, making it difficult to draw any conclusions about the clinical relevance of the findings.
A few more considerations are needed: first of all, different species display similar activities such as antioxidants and anti-inflammatory properties, despite being characterised by different genes, habitat, physiological needs and eating habits, making it difficult to associate the specific functional role to the different species. Studies assessing the functional properties of different species characterised by dietary habits and environment, as well as the effect of different feedings, will help us to understand the variables influencing the functional properties of the insects.
Secondly, the majority of the studies tested extracts without identifying the compounds responsible for the functional effect or without testing sub-fractions with different chemical composition. However, although functional properties of the edible insects might be related to their variegate molecular composition, as occurs for foods, the identification of the single molecules is a necessary step for nutraceutical or pharmacological purposes.
Thirdly, the evidence from in vitro, cellular and animal models on the functional properties reviewed in this manuscript is based on robust and reliable markers with a statistical significance. However, the scarce evidence in humans highlights the urgent need for long-term dietary intervention trials to endorse the role of edible insects as functional foods. The step of human consumption is critical because there is no information on the bioavailability of functional molecules from edible insects in body fluids following consumption. Moreover, it would be interesting to understand the effect of intestinal microbiota on molecules from the digestion processes of edible insects and if the arising metabolites are endowed with functional properties. In this view, it would be interesting to develop observational or epidemiological studies assessing biomarkers related to antioxidant, inflammatory and immune status as well as microbiota analyses in populations where insects are a part of the usual diet.
The inclusion of edible insects in the category of functional food might open a new scenario for food industries through the enrichment of food with proteins, extracts or flour obtained from edible insects, representing a novel aspect with scientific, commercial and social impacts on society in the following years. However, for such an approach to be efficacious, it is important to investigate consumers’ acceptance, mainly in Western countries, of insect-based foods(Reference Skotnicka, Karwowska and Kłobukowski74). This is a process that requires time and campaigning of information for consumers, focusing on the importance of reducing the ecological impact of animal-based products with more sustainable and functional foods.
Conclusions
In conclusion, based on the available body of evidence, mainly in vitro, cellular and animal studies, edible insects are a promising source of bioactive ingredients endowed with antioxidant and anti-inflammatory properties, involved in the modulation of glucose and lipid metabolism, potentially leading to health benefits. However, dietary intervention trials are urgently needed to confirm and define the efficacy of edible insects as functional foods in humans, confirming the promising evidence from in vitro and animal models. We think that this review could promote research on the functional properties of edible insects, amplifying our knowledge on this topic and ensuring that correct information is relayed from the media to consumers.
Author contributions
M.S. conceived the topic of the review and supervised the work. V.D.A. wrote the initial draft. N.B., G.S. and C.D.M. revised the work. All authors approved the submitted version.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.










