No CrossRef data available.
Article contents
Atomic Transport by Ballistic Atomic Mixing Effect in Bilayer Structure
Published online by Cambridge University Press: 22 February 2011
Abstract
A dynamic Monte-Carlo simulation (MCS) program, containing not only collisional mixing but also sputtering effects, has been used to elucidate the dynamic mixing processes and the atomic transport of constituents in Al-Pd bilayer systems. MCS results reveal that the preferential inward displacement of the top layer element dominates, and that there is an enhancement of the inward displacement when the heavier element is in the top layer. The inward displacement is controlled by both of an anisotropic and isotropic atomic transport. The anisotropic term is caused by the primary recoil of atoms, which has a characteristic of φ(ion dose) dependence, and the isotropic term is associated with the random cascade motion, which has a 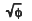 dependence. However the outward displacement is governed by only the isotropic motion, thus the inward displacement always dominates over the outward motion, which leads to a preferential displacement of top layer element to the bottom layer.
dependence. However the outward displacement is governed by only the isotropic motion, thus the inward displacement always dominates over the outward motion, which leads to a preferential displacement of top layer element to the bottom layer.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1994




