No CrossRef data available.
Article contents
Phase Relations of the Uranyl Oxide Hydrates and their Relevance to the Disposal of Spent Fuel
Published online by Cambridge University Press: 28 February 2011
Abstract
Uranyl oxide hydrates, formed by the alteration of uraninite, are natural analogues for the long-term corrosion products of spent fuel in a geologic repository under oxidizing conditions. The uranyl oxide hydrates may be represented by the general formula:
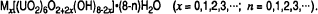
Pb-bearing hydrates require the addition of a neutral uranyl group into the structural sheet (UO2(OH)2) for each interlayer Pb ion. Distortion of the structure associated with the additional uranyl groups is reduced by replacing two structural hydroxyls with a structural oxygen and a molecular water. The general formula for the Pb-uranyl oxide hydrates is:
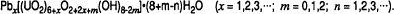
This hypothesis explains the paragenetic sequences:
1) schoepite ➛ billietite ➛ protasite ➛ bauranoite
2) schoepite ➛ vandendriesscheite ➛ fourmarierite ➛ masuyite ➛ wölsendorfite
3) schoepite ➛ vandendriesscheite ➛ fourmarierite ➛ ± masuyite ➛ sayrite ➛ curite, and indicates that, under relatively high pH conditions, schoepite will not be the long-term solubility-controlling phase for uranium in uranium-rich groundwaters.
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1991




