Article contents
Variations in electronic states of coumarin hexanethiolate-labeled i-Au25 and bi-Au25 clusters
Published online by Cambridge University Press: 27 August 2019
Abstract
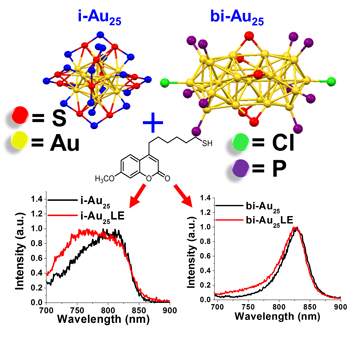
 ${\rm Au}_{25}\lpar {{\rm C}_6{\rm H}_{14}{\rm S}} \rpar_{18}{}^-$ icosahedron and [Au25(PPh)10(C6H14S)5Cl2]2+ bi-icosahedron clusters were synthesized. Ligand exchange reactions were carried out with a new coumarin-derived fluorophore (Cou-SH) to label both clusters. Labeled and unlabeled Au25 were compared and the changes in the electronic structure were determined. The labeled clusters showed marked changes in electronic states, as evidenced by the quenching in the UV region and enhancement in the near infrared. The quantum yield from Cou-SH decreased and the quantum yield from the labeled Au25 increased. Second, the authors observed changes in the electrochemical band gap.
${\rm Au}_{25}\lpar {{\rm C}_6{\rm H}_{14}{\rm S}} \rpar_{18}{}^-$ icosahedron and [Au25(PPh)10(C6H14S)5Cl2]2+ bi-icosahedron clusters were synthesized. Ligand exchange reactions were carried out with a new coumarin-derived fluorophore (Cou-SH) to label both clusters. Labeled and unlabeled Au25 were compared and the changes in the electronic structure were determined. The labeled clusters showed marked changes in electronic states, as evidenced by the quenching in the UV region and enhancement in the near infrared. The quantum yield from Cou-SH decreased and the quantum yield from the labeled Au25 increased. Second, the authors observed changes in the electrochemical band gap.
- Type
- Research Letters
- Information
- Copyright
- Copyright © The Author(s) 2019
Footnotes
These authors contributed equally to this work.
References
- 1
- Cited by




