Article contents
Fabrication of optically active fiber mats via melt electrospinning
Published online by Cambridge University Press: 13 August 2018
Abstract
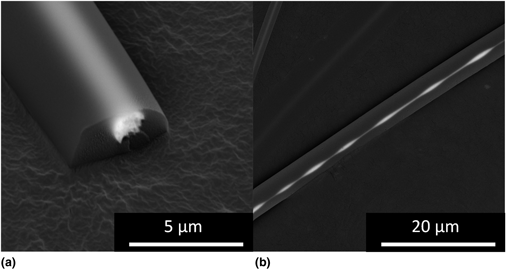
Melt electrospinning is a facile fabrication technique that can be utilized in the creation of microfibers without the use of solvent and with good control over feature placement. The available thermal energy of the melt electrospinning technique is often only utilized in the formation of the polymer melt but can also be used to thermodynamically drive chemical reactions. In this study, hybrid perovskite microcrystallites are synthesized in the polymer melt and electrospun to form composite microfibers. Unique hybrid perovskite microstructures were studied, elucidating mechanisms of formation at work in the polymer melt.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 3
- Cited by




