Article contents
Conductive polymer binder and separator for high energy density lithium organic battery
Published online by Cambridge University Press: 02 September 2019
Abstract
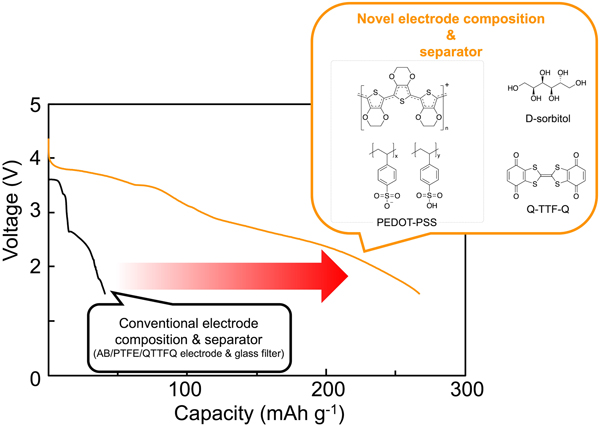
The practical realization of rechargeable organic batteries is stalled by their low electron conductivity, which limits the organic-active material content in the electrode composite and results in a low net electrode energy density. Additionally, the dissolution of active materials into the electrolyte causes a short cycle life. In this study, a conductive polymer mixture, poly(3,4-ethylenedioxythiophene)/polystyrenesulfonate, containing a small amount of sugar alcohol was used as the binder and separator in a rechargeable organic battery. Consequently, the active material content was increased up to 80 wt%, and the cycle life was extended.
- Type
- Research Letters
- Information
- Copyright
- Copyright © The Author(s) 2019
References
- 6
- Cited by




