Article contents
Amine modifications of polypropylene films by gamma radiation to be applied in cell cultures
Published online by Cambridge University Press: 26 September 2019
Abstract
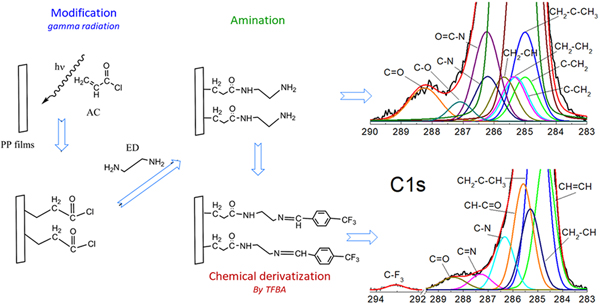
Development of biomaterials with primary amine surfaces is very important for the study of some cells of the immune systemuch as macrophages. Currently, the modification can be carried out by physical or chemical methods with several disadvantages due to the presence of additives or subproducts in the system. To overcome this problem, modified polypropylene (PP) films were synthesized by gamma radiation. In this work, radiation grafting of acryloyl chloride onto PP has been employed to form an acyl chloride. Then, the radiation-grafted films were reacted with ethylenediamine in several solvents to obtain the different concentration of the primary amine. The surface amine concentration was determined by derivatization with 4-trifluoromethyl benzaldehyde and characterized by x-ray photoelectron spectroscopy (N/C ratios), Fourier transform infrared spectroscopy with attenuated total reflection, contact angle, scanning electron microscopy, atomic force microscopy, and elementary analysis. The stability of the amines was measured up to 90 days, without the occurrence of aging as was found by plasma modification.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 1
- Cited by





