Article contents
Graphene oxide film as a template for the creation of three-dimensional lamellar metal oxides and reduced graphene oxide/metal oxide hybrids
Published online by Cambridge University Press: 19 November 2014
Abstract
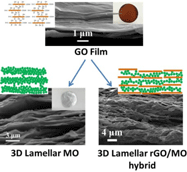
Here we report a general method for the synthesis of layered inorganic nanocrystalline materials using graphene oxide (GO) film as the template. Free-standing three-dimensional (3D) lamellar ZnO, α-Fe2O3, and reduced GO/ZnO hybrid structures were synthesized as examples. Such layered structures could also be exfoliated to obtain 2D assembled nanocrystal microsheets. The abundant nucleation sites on the GO surface and the compact stacking of GO platelets made it possible to tightly control metal oxide crystal size (~15 nm), alter preferential crystal growth direction, and assemble the nanocrystals into sheets, as confirmed by multiple characterization techniques.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
- 5
- Cited by




