Article contents
Effects of nanoporous Au on ATP synthase
Published online by Cambridge University Press: 14 January 2020
Abstract
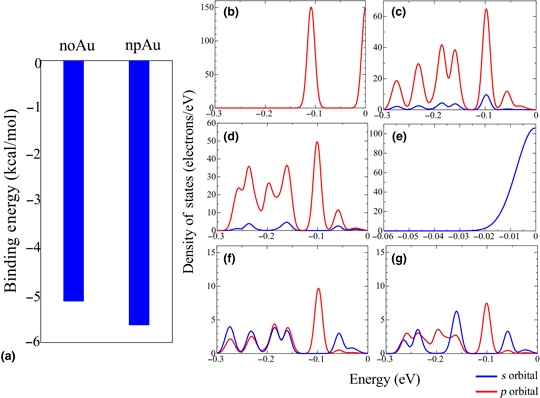
Nanoporous Au shows the antimicrobial activity without the release of ROS. The cell wall is hyperpolarized by npAu. Hence, the hyperpolarized cell wall may affect ATP synthase, leading to the bacterial death. In the present work, the effects of the hyperpolarized cell wall on the structure and functions of Asp61 in ATP synthase are investigated by molecular dynamics simulations and first-principles calculations. The simulations suggest that the Asp61 is more negatively hyperpolarized, which is due to the strengthened O–H bond in Asp61, in interacting with the hyperpolarized cell wall, which results in the disturbance of proton transport in ATP synthase.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 1
- Cited by





