Article contents
Deciphering charge-storage mechanisms in 3D MnOx@carbon electrode nanoarchitectures for rechargeable zinc-ion cells
Published online by Cambridge University Press: 29 January 2019
Abstract
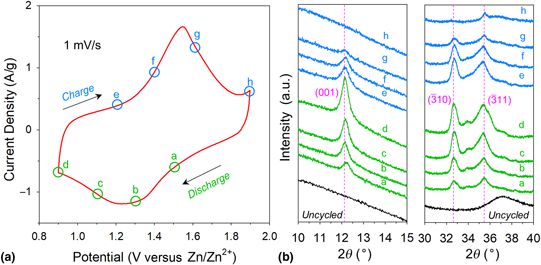
We previously demonstrated that electrode architectures comprising nanoscale birnessite-like MnOx affixed to three-dimensional carbon nanofoam (CNF) scaffolds offer performance advantages when used as cathodes in rechargeable zinc-ion cells. To discern chemical and physical changes at the MnOx@CNF electrode upon deep charge/discharge in aqueous Zn2+-containing electrolytes, we deploy electroanalytical methods and ex situ characterization by microscopy, elemental analysis, x-ray photoelectron spectroscopy, x-ray diffraction, and x-ray pair distribution function analyses. Our findings verify that redox processes at the MnOx are accompanied by reversible precipitation/dissolution of crystalline zinc hydroxide sulfate (Zn4(OH)6(SO4)·xH2O), mediated by the more uniformly reactive electrode structure inherent to the CNF scaffold.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 7
- Cited by




