Article contents
Some high-pressure pyroxenes
Published online by Cambridge University Press: 05 July 2018
Summary
Microprobe analyses of Ca-rich pyroxenes crystallized in the melting ranges of a magnesian alkali basalt, a transitional basalt, an olivine tholeiite, a tholeiitic andesite, and an augite leucitite at pressures between 8 and 45 kb show complex variation. Ca-poor pyroxene precipitated only from the alkali basalt at pressures between 14 and 18 kb. Pyroxene falling near the Di-Hed join in the pyroxene quadilateral formed at all pressures and temperatures from the leucitite, whereas ‘Ca-rich’ pyroxene crystallizing from the other four compositions was Ca-poor augite to sub-calcic augite. The liquidus Ca-rich pyroxenes all show rising Al and Na and falling Ti with increasing pressure and temperature. Other elements show complex behaviour; all but the leucitite pyroxenes tend to make temporary excursions of solid solution towards Ca-poor pyroxene at intermediate pressures, returning to more Ca-rich compositions at high pressures. At sub-liquidus temperatures Na and Ti consistently rise with falling T at constant P and also with rising P at constant T in these pyroxenes. The behaviour of the other elements in these circumstances depends on the nature of the coexisting phases.
Fe/Mg distribution between Ca-rich pyroxene and liquid, in the form
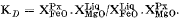
has a constant value of 0.29 for three separate bulk compositions at widely differing temperatures and pressures. Distribution coefficients for Mg and Fe between pyroxenes and coexisting garnets at high pressures are very similar to those found in garnet pyroxenite xenoliths from Oahu, Hawaii. Systematic shifts in the apparent stoichiometry (all Fe taken as Fe2+) of the augite leucitite pyroxenes are thought to indicate that they have considerable Fe3+ contents at low pressure, decreasing as P rises. If so, they show a strong negative correlation between Na and Fe3+, which negates the customary practice of forming acmite before jadeite component when recalculating the analyses of high-pressure pyroxenes.
The sets of pyroxenes crystallized from each composition show consistent trends when plotted on such diagrams as jadeite vs Ca-Tschermak's ‘molecule’, which have often been used in attempts to discriminate natural pyroxenes formed in differing P-T environments. However, these new data show clearly that the bulk chemistry of the magma has a predominating influence on the composition of the pyroxenes crystallizing from it. Unless it is certain that a suite of natural pyroxenes have all precipitated from the same magma, it is probably pointless to attempt to deduce the relative P-T conditions of their formation from their major element chemistry.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1974
References
- 156
- Cited by




