No CrossRef data available.
Article contents
Mangani-eckermannite, NaNa2(Mg4Mn3+)Si8O22(OH)2, a new amphibole from Tanohata Mine, Iwate Prefecture, Japan
Published online by Cambridge University Press: 10 August 2023
Abstract
Mangani-eckermannite, ideally NaNa2(Mg4Mn3+)Si8O22(OH)2, is a new member of the amphibole supergroup found at Tanohata Mine, Shimohei District, Iwate Prefecture, Japan. It occurs as prismatic crystals up to 0.3 × 0.2 mm and their aggregates up to 1 mm intergrown with braunite, vittinkiite and quartz. Mangani-eckermannite is cherry-red to very dark red and reddish-brown in thicker grains. It is translucent with a pinkish white streak and vitreous lustre. It is brittle, fracture is stepped along crystal elongation and uneven across a crystal. Cleavage is perfect on {110}. Mohs hardness is 6. Dmeas = 3.16(2) and Dcalc = 3.186 g/cm3. The mineral is optically biaxial (–), with α = 1.645(3), β = 1.668(2), γ = 1.675(3) (589 nm); 2Vmeas = 60(10)°, 2Vcalc = 57°. The empirical formula derived from electron microprobe analysis, secondary-ion mass spectrometry and single-crystal structure refinement and calculated on the basis of 24 (O+OH) atoms per formula unit (apfu) is A(Na0.74K0.24□0.02)Σ1.00 B(Na1.52Ca0.24Mn2+0.24)Σ2.00 C(Mg2.54Mn2+1.45Mn3+0.71Fe3+0.26Ti0.04)Σ5.00 T(Si7.97Al0.03)Σ8.00O22W[(OH)1.52O0.48]Σ2.00. Mangani-eckermannite is monoclinic, space group C2/m, a = 9.9533(4), b = 18.1440(7), c = 5.2970(2) Å, β = 103.948(4)°, V = 928.39(6) Å3 and Z = 2. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 8.52(100)(110); 4.54(25)(040); 3.41(29)(131); 3.16(23)(310,201); 2.721(37)(151); 2.533(26)($\bar{2}$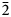 02). The crystal structure was refined to R1 = 0.0264 for 1001 independent reflections with I > 2σ(I). The place of mangani-eckermannite in the nomenclature of the amphibole supergroup is discussed and the status of mangano-ferri-eckermannite as a valid mineral species and successor of ‘kôzulite’ is questioned.
02). The crystal structure was refined to R1 = 0.0264 for 1001 independent reflections with I > 2σ(I). The place of mangani-eckermannite in the nomenclature of the amphibole supergroup is discussed and the status of mangano-ferri-eckermannite as a valid mineral species and successor of ‘kôzulite’ is questioned.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2023. Published by Cambridge University Press on behalf of The Mineralogical Society of the United Kingdom and Ireland
Footnotes
Associate Editor: Mihoko Hoshino




