Article contents
I. Whiteite, a new species, and a proposed nomenclature for the jahnsite-whiteite complex series. II. New data on xanthoxenite. III. Salmonsite discredited
Published online by Cambridge University Press: 05 July 2018
Summary
Whiteite, Ca(Fe,Mn)2+Mg2Al2(OH)2 (H2O)8[PO4]4, a 14·90(4) Å, b 6·98(2) Å, c 10·13(2) Å, β 113° 07(10)′, Z = 2, space group P2/a, α 1·580(5), β 1·585(5), γ 1·590(5), 2V 40–50°, specific gravity 2·58, is a new species from the Ilha de Taquaral, Minas Gerais, Brazil. It is the Al3+-analogue of jahnsite. The mineral occurs as up to 5 mm tan crystals flattened on {001}. Twinning by reflection on {001} leads to pseudoorthorhombic development. Rather pure material also occurs from Blow River, Yukon Territory, Canada.
For the general formula XM(1)M(2)2M(3)2(OH)2 (H2O)8[PO4]4, it is proposed that for M(3), Al3+ > Fe3+, the established members of the series are whiteite—(CaFe2+Mg) and whiteite—(Mn2+Fe2+Mg); and for Fe3+ > Al3+, jahnsite—(CaMn2+Mg), jahnsite—(CaMn2+Fe2+), and possibly jahnsite—(Mn2+Mn2+Mn2+).
Xanthoxenite of Laubmann and Steinmetz (1920) is probably stewartite (in part) on the basis of morphological, optical, physical, and paragenetic evidence. The xanthoxenite of Frondel (1949) is proposed as the species type. It is triclinic, P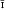 or P1, a 6·70(4) Å, b8·85(4) Å, c 6·54(3) Å, α 92·1(2)°, β 110·2(2)°, γ 93·2(2)°, Z = 1 for composition
or P1, a 6·70(4) Å, b8·85(4) Å, c 6·54(3) Å, α 92·1(2)°, β 110·2(2)°, γ 93·2(2)°, Z = 1 for composition  .
.
Salmonsite, c. 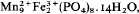 from Pala, California, is shown to be an intimate mixture of hureaulite and jahnsite on the basis of calculated and observed powder patterns and on reinterpretation of the original chemical analysis published by Schaller (1912). It is a breakdown product resulting from oxidation of Fe2+ in the original hureaulite (‘palaite’) along with further aquation followed by fine-grained recrystallization. The reaction proposed is:
from Pala, California, is shown to be an intimate mixture of hureaulite and jahnsite on the basis of calculated and observed powder patterns and on reinterpretation of the original chemical analysis published by Schaller (1912). It is a breakdown product resulting from oxidation of Fe2+ in the original hureaulite (‘palaite’) along with further aquation followed by fine-grained recrystallization. The reaction proposed is:
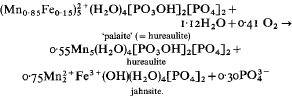
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1978
Footnotes
Died 6 June 1978.
References
- 25
- Cited by


