Article contents
Gurzhiite, Al(UO2)(SO4)2F⋅10H2O, a new uranyl sulfate mineral with a chain structure from the Bykogorskoe deposit, Northern Caucasus, Russia
Published online by Cambridge University Press: 12 May 2022
Abstract
Gurzhiite, ideally Al(UO2)(SO4)2F⋅10H2O, is a new uranyl sulfate mineral from the Bykogorskoe U deposit, Northern Caucasus, Russia. It occurs as fine-grained aggregates forming veinlets up to 50 cm long in cracks of the brecciated rock. Gurzhiite aggregates are composed of small bladed crystals up to 0.1 mm across. Associated minerals include khademite and quartz. Gurzhiite is pale yellow in crystals, lemon yellow in aggregates, transparent with a vitreous lustre and a white streak. It is brittle and has an irregular fracture. Cleavage is good on {001}. The new mineral exhibits a bright yellow–green fluorescence under both longwave and shortwave UV radiation. Mohs hardness is ~2. Dmeas = 2.52(3) g/cm3 and Dcalc = 2.605 g/cm3. The mineral is biaxial (–) with α = 1.528(3), β = 1.538(2), γ = 1.544(3) (589 nm); 2Vmeas= 80(10)° and 2Vcalc = 75.1°. The empirical formula calculated on the basis of 21(O + F) atoms per formula unit (apfu) is Al0.92Zn0.05Fe3+0.03Na0.03U0.95S2.00O9.85F0.99⋅10.16H2O. Gurzhiite is triclinic, with space group P$\bar{1}$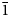 , a = 7.193(2), b = 11.760(2), c = 11.792(2) Å, α = 67.20(3), β = 107.76(3), γ = 89.99(3)°, V = 867.7(4) Å3 and Z = 2. The five strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 10.24(100)(001); 5.40(14)($\bar{ 1}\bar{1}$
, a = 7.193(2), b = 11.760(2), c = 11.792(2) Å, α = 67.20(3), β = 107.76(3), γ = 89.99(3)°, V = 867.7(4) Å3 and Z = 2. The five strongest lines of the powder X-ray diffraction pattern [d, Å (I, %)(hkl)] are: 10.24(100)(001); 5.40(14)($\bar{ 1}\bar{1}$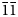 1); 5.11(54)(002); 3.405(11)($\bar{2}$
1); 5.11(54)(002); 3.405(11)($\bar{2}$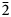 11); and 3.065(11)($\bar{1}\bar{1}$
11); and 3.065(11)($\bar{1}\bar{1}$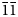 3). The crystal structure of gurzhiite is based upon uranyl sulfate chains of the same type as in bobcookite and svornostite. Between the chains are two types of Al-octahedra – Al1(H2O)6 and Al2F2(H2O)4. The entire structure stability is maintained by a complex network of H bonds. The new mineral honours Russian mineralogist and crystallographer Dr. Vladislav V. Gurzhiy in recognition for his contributions to uranium mineralogy and crystallography.
3). The crystal structure of gurzhiite is based upon uranyl sulfate chains of the same type as in bobcookite and svornostite. Between the chains are two types of Al-octahedra – Al1(H2O)6 and Al2F2(H2O)4. The entire structure stability is maintained by a complex network of H bonds. The new mineral honours Russian mineralogist and crystallographer Dr. Vladislav V. Gurzhiy in recognition for his contributions to uranium mineralogy and crystallography.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Daniel Atencio
References
- 2
- Cited by




