Article contents
Drugmanite, Pb2(Fe3+,Al) (PO4)2(OH) · H2O, a new mineral from Richelle, Belgium
Published online by Cambridge University Press: 05 July 2018
Summary
Drugmanite occurs as rare colourless transparent platy crystals, up to 0.2 mm, aggregated in bunches, in vugs of a mineralized and silicified limestone. Hardness < 6. Crystals monoclinic, forms {001} {110}, parameters from indexed X-ray powder pattern (and monocrystal measurements): a = 11.110 (11.111) Å, b = 7.976 (7.986), c = 4.644 (4.643), β = 90°18′ (90°.41°). Space group P21/a with Z = 2 giving Dcalc = 5.55. Strongest lines are 4.63 Å (9), 3.752 (IO), 3.350 (8), 3.247 (8), 2.912 (9). Mean refractive index 1.88 from reflectance measurements. Strong dispersion r < v, optic axial plane // (olo), 2Vα = 33±2°. Electron microprobe analysis gave P 8.89, Al 0.85, Fe 6.19, Pb 59.76%, leading to Pb4.02
(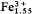 Al0.45)P4.00O17.02·3H2O or Pb2(
Al0.45)P4.00O17.02·3H2O or Pb2(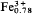 Al0.22) (PO4)2 (OH)·H2O. Associated minerals are pyromorphite, anglesite, corkite and phosphosiderite. Named for J. Drugman, Belgian mineralogist (1875–1950).
Al0.22) (PO4)2 (OH)·H2O. Associated minerals are pyromorphite, anglesite, corkite and phosphosiderite. Named for J. Drugman, Belgian mineralogist (1875–1950).
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1979
References
- 2
- Cited by




