Article contents
Chiral edge-shared octahedral chains in liskeardite, [(Al,Fe)32(AsO4)18(OH)42(H2O)22]·52H2O, an open framework mineral with a pharmacoalumite-related structure
Published online by Cambridge University Press: 05 July 2018
Abstract
The type specimen of liskeardite, (Al, Fe)3AsO4(OH)6·5H2O, from the Marke Valley Mine, Liskeard District, Cornwall, has been reinvestigated. The revised composition from electron microprobe analyses and structure refinement is [Al29.2Fe2.8(AsO4)18(OH)42(H2O)22]·52H2O. The crystal structure was determined using synchrotron data collected on a 2 μm diameter fibre at 100 K. Liskeardite has monoclinic symmetry, space group I2, with the unit-cell parameters a = 24.576(5), b = 7.754(2) Å, c = 24.641(5) Å, and β = 90.19(1)º. The structure was refined to R = 0.059 for 9769 reflections with I > 3σ(I). It is of an open framework type in which intersecting polyhedral slabs parallel to (101) and (10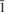 ) form 17.4 Å × 17.4 Å channels along [010], with water molecules occupying the channels. Small amounts (<1 wt.%) of Na, K and Cu are probably adsorbed at the channel walls The framework comprises columns of pharmacoalumite-type, intergrown with chiral chains of six cis edge-shared octahedra. It can be described in terms of cubic close packing, with vacancies at both the anion and cation sites. The compositional and structural relationships between liskeardite and pharmacoalumite are discussed and a possible mechanism for liskeardite formation is presented.
) form 17.4 Å × 17.4 Å channels along [010], with water molecules occupying the channels. Small amounts (<1 wt.%) of Na, K and Cu are probably adsorbed at the channel walls The framework comprises columns of pharmacoalumite-type, intergrown with chiral chains of six cis edge-shared octahedra. It can be described in terms of cubic close packing, with vacancies at both the anion and cation sites. The compositional and structural relationships between liskeardite and pharmacoalumite are discussed and a possible mechanism for liskeardite formation is presented.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2013
References
- 9
- Cited by


